1. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003; 24:668–93.

2. Lee PA. Central precocious puberty. An overview of diagnosis, treatment, and outcome. Endocrinol Metab Clin North Am. 1999; 28:901–18.
3. Partsch CJ, Heger S, Sippell WG. Management and outcome of central precocious puberty. Clin Endocrinol (Oxf ). 2002; 56:129–48.

4. Lahlou N, Carel JC, Chaussain JL, Roger M. Pharmacokinetics and pharmacodynamics of GnRH agonists: clinical implications in pediatrics. J Pediatr Endocrinol Metab. 2000; 13 Suppl 1:723–37.

5. Kim EY. Long-term effects of gonadotropin-releasing hormone analogs in girls with central precocious puberty. Korean J Pediatr. 2015; 58:1–7.

6. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016; 4:265–74.

7. Arrigo T, De Luca F, Antoniazzi F, Galluzzi F, Segni M, Rosano M, et al. Reduction of baseline body mass index under gonadotropin-suppressive therapy in girls with idiopathic precocious puberty. Eur J Endocrinol. 2004; 150:533–7.

8. De Bond JA, Smith JT. Kisspeptin and energy balance in reproduction. Reproduction. 2014; 147:R53–63.

9. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008; 51:1–25.

10. Greulich W, Pyle S. Radiologic atlas of skeletal development of the hand and wrist. 2nd ed. Redwood City (CA): Stanford University Press;1959.
11. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952; 40:423–41.

12. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969; 44:291–303.

13. Tanner JM, Goldstein H, Whitehouse RH. Standards for children's height at ages 2-9 years allowing for heights of parents. Arch Dis Child. 1970; 45:755–62.
14. Mogensen SS, Aksglaede L, Mouritsen A, Sørensen K, Main KM, Gideon P, et al. Diagnostic work-up of 449 consecutive girls who were referred to be evaluated for precocious puberty. J Clin Endocrinol Metab. 2011; 96:1393–401.

15. Kim HK, Kee SJ, Seo JY, Yang EM, Chae HJ, Kim CJ. Gonadotropin-releasing hormone stimulation test for precocious puberty. Korean J Lab Med. 2011; 31:244–9.

16. Health Insurance Review & Assessment Service. The early detection of precocious puberty is important [Internet]. Seoul: Health Insurance Review & Assessment Service;c2013. [cited 2016 Sep 14]. Available from:
http://www.hira.or.kr.
17. Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015; 175:1412–3.

18. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006; 295:1549–55.

19. Heger S, Körner A, Meigen C, Gausche R, Keller A, Keller E, et al. Impact of weight status on the onset and parameters of puberty: analysis of three representative cohorts from central Europe. J Pediatr Endocrinol Metab. 2008; 21:865–77.

20. He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001; 49:244–51.

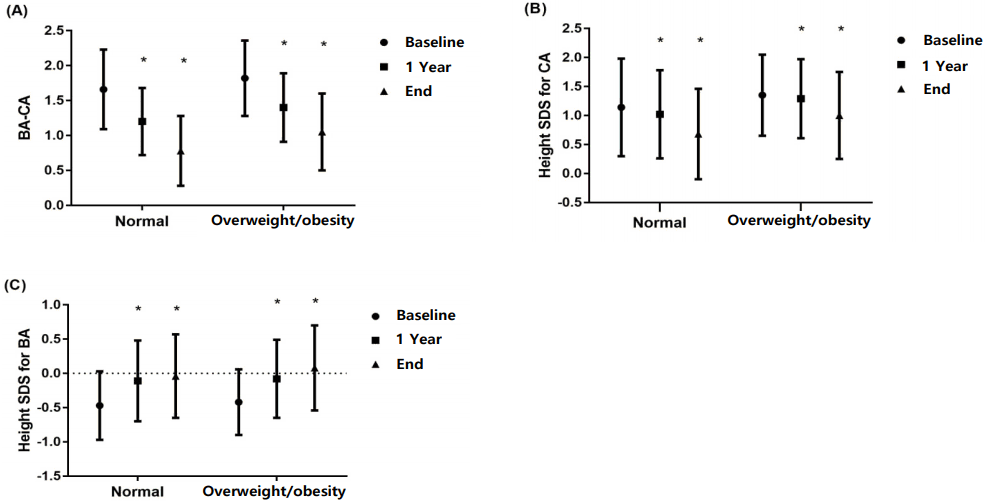
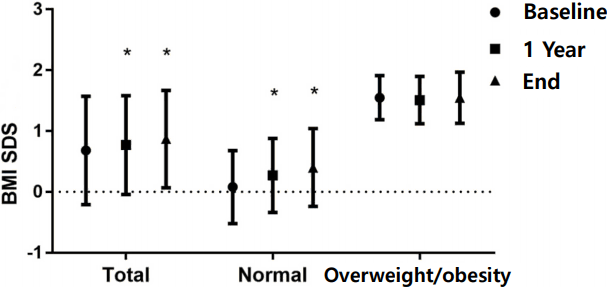
21. Yoon JY, Kang MJ, Kim SY, Seo JY, Yang SW, Lee YA, et al. The relationship between initial body mass index and body mass index after one year of gonadotropin-releasing hormone agonist therapy in idiopathic true precocious puberty girls. J Korean Soc Pediatr Endocrinol. 2011; 16:165–71.

22. Partsch CJ, Heger S, Sippell WG. Treatment of central precocious puberty: lessons from a 15 years prospective trial. German Decapeptyl Study Group. J Pediatr Endocrinol Metab. 2000; 13 Suppl 1:747–58.

23. Arrigo T, Cisternino M, Galluzzi F, Bertelloni S, Pasquino AM, Antoniazzi F, et al. Analysis of the factors affecting auxological response to GnRH agonist treatment and final height outcome in girls with idiopathic central precocious puberty. Eur J Endocrinol. 1999; 141:140–4.

24. Gyon Y, Yun YJ, Kim YD, Han HS. Age at menarche and near final height after treatment with gonadotropinreleasing hormone agonist alone or combined with growth hormone in Korean girls with central precocious puberty. Clin Pediatr Endocrinol. 2015; 24:175–83.

25. Heger S, Partsch CJ, Sippell WG. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty: final height, body proportions, body composition, bone mineral density, and reproductive function. J Clin Endocrinol Metab. 1999; 84:4583–90.

26. Shiasi Arani K, Heidari F. Gonadotropin-releasing hormone agonist therapy and obesity in girls. Int J Endocrinol Metab. 2015; 13:e23085.

27. Paterson WF, McNeill E, Young D, Donaldson MD. Auxological outcome and time to menarche following longacting goserelin therapy in girls with central precocious or early puberty. Clin Endocrinol (Oxf). 2004; 61:626–34.

28. Wolters B, Lass N, Reinehr T. Treatment with gonadotropinreleasing hormone analogues: different impact on body weight in normal-weight and overweight children. Horm Res Paediatr. 2012; 78:304–11.

29. Lee HS, Yoon JS, Roh JK, Hwang JS. Changes in body mass index during gonadotropin-releasing hormone agonist treatment for central precocious puberty and early puberty. Endocrine. 2016; 54:497–503.

30. Pich J, Bibiloni Mdel M, Pons A, Tur JA. Weight selfregulation process in adolescence: the relationship between control weight attitudes, behaviors, and body weight status. Front Nutr. 2015; 2:14.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download