Abstract
Epiploic appendagitis (EA) is an uncommon intraabdominal pathology resulting in transient, localized pain. The condition is caused by ischemia of one of the epiploic appendages, which are distributed axially along the length of the colon. EA is often mistaken for other more common etiologies of an acute abdomen. Generally, the patients experience focal abdominal pain with no further symptoms or laboratory abnormalities. The authors encountered a 79-year-old male with severe sepsis and acute respiratory failure requiring intubation. He recovered rapidly after the identification and removal of a single EA. This paper reports the first case of EA leading to the systemic dysregulation of sepsis.
Epiploic appendages are serosa-covered, fat-filled outpouchings found almost exclusively in the large intestine.1,2 Their physiological roles are uncertain, but they are presumed to have a defensive and protective capacity analogous to the greater omentum.3,4 Epiploic appendagitis (EA) occurs when an epiploic appendage experiences ischemia through either torsion of the appendage stalk or thrombosis of the central draining vein.5 The documented risk factors include male sex, obesity, intensive strenuous exercise, and the presence of a hernia.6 The condition is rare, with 8.8 cases per 100,000 patients reported annually and often occurring in the fourth to fifth decade of life7 The diagnosis is often benign, leading to abrupt focal abdominal pain, but lacking abdominal rigidity or notable guarding, typically without leukocytosis or other systemic signs.8 Effective treatment commonly involves conservative management with non-steroidal anti-inflammatory medications, with surgical intervention only required in special circumstances. Some operative indications include recurrent symptoms, complications (e.g., abscess formation, bowel obstruction, or intussusception), or severe disease resulting in progressive pain, high fever, or poor toleration of oral intake.9 EA is often underdiagnosed because of its rarity and mimicry of alternative abdominal pathologies. Prompt identification can help avoid unnecessary surgical interventions. On the other hand, in extremely rare cases, EA is capable of causing a critical decline in select patients, as this case demonstrates.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
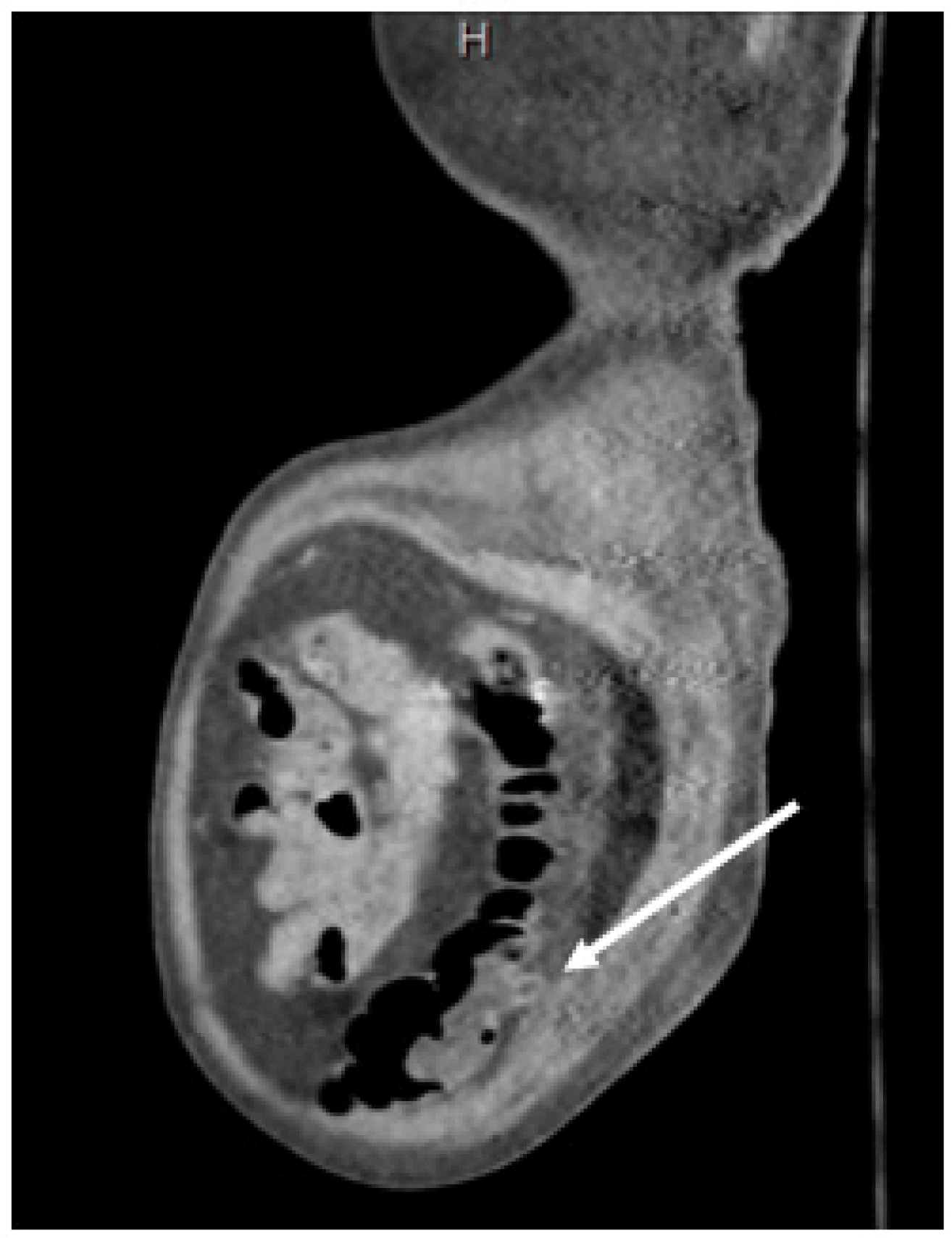
A 79-year-old male arrived by ambulance complaining of a four-day history of severe dizziness, anorexia, and dyspepsia. The physical examination was remarkable for obesity and mild epigastric pain to deep palpation, with his vital signs all within the normal limits. Laboratory analyses showed only slightly elevated troponins of 0.141 ng/mL and BUN/creatine of 41/1.4 mg/dL. The patient was admitted for workup and the treatment of non-ST elevation myocardial infarction and acute kidney injury. During his hospital stay, he developed abdominal pain that was initially localized to the left lower quadrant of the abdomen but gradually worsened, with tenderness developing in all abdominal quadrants. The patient’s mental activity declined steadily, with the onset of confusion with progression to lethargy and obtundation over 36 hours. During this rapid decline, the patient also developed acute hypercapnic hypoxic respiratory failure requiring bilevel positive airway pressure and was upgraded to the intensive care unit for close monitoring. Upon arrival at the unit, his vital signs showed a peak respiratory rate of 33 breaths/minute. Significant laboratory analyses revealed the following: lactate level of 2.24 mmol/L, an erythrocyte sedimentation rate of 88 mm/hr, C-reactive protein level of 27.5 mg/dL, normal white blood cell count, worsening BUN/creatinine level of 60/1.9 mg/dL, and an increasing troponin level of 0.285 ng/mL. The patient was treated according to the sepsis protocol with isotonic fluids and piperacillin/tazobactam 3 gm/0.375 gm every eight hours. An ultrasound of the right upper quadrant was performed considering the patient’s precipitous decline and acute abdomen, which revealed cholelithiasis without evidence of cholecystitis. A computed tomography (CT) scan of the abdomen and pelvis with contrast revealed inflammatory changes posterior to the left side of the colon with a single diverticulum visible, which is indicative of diverticulitis versus nonspecific colitis (Fig. 1).
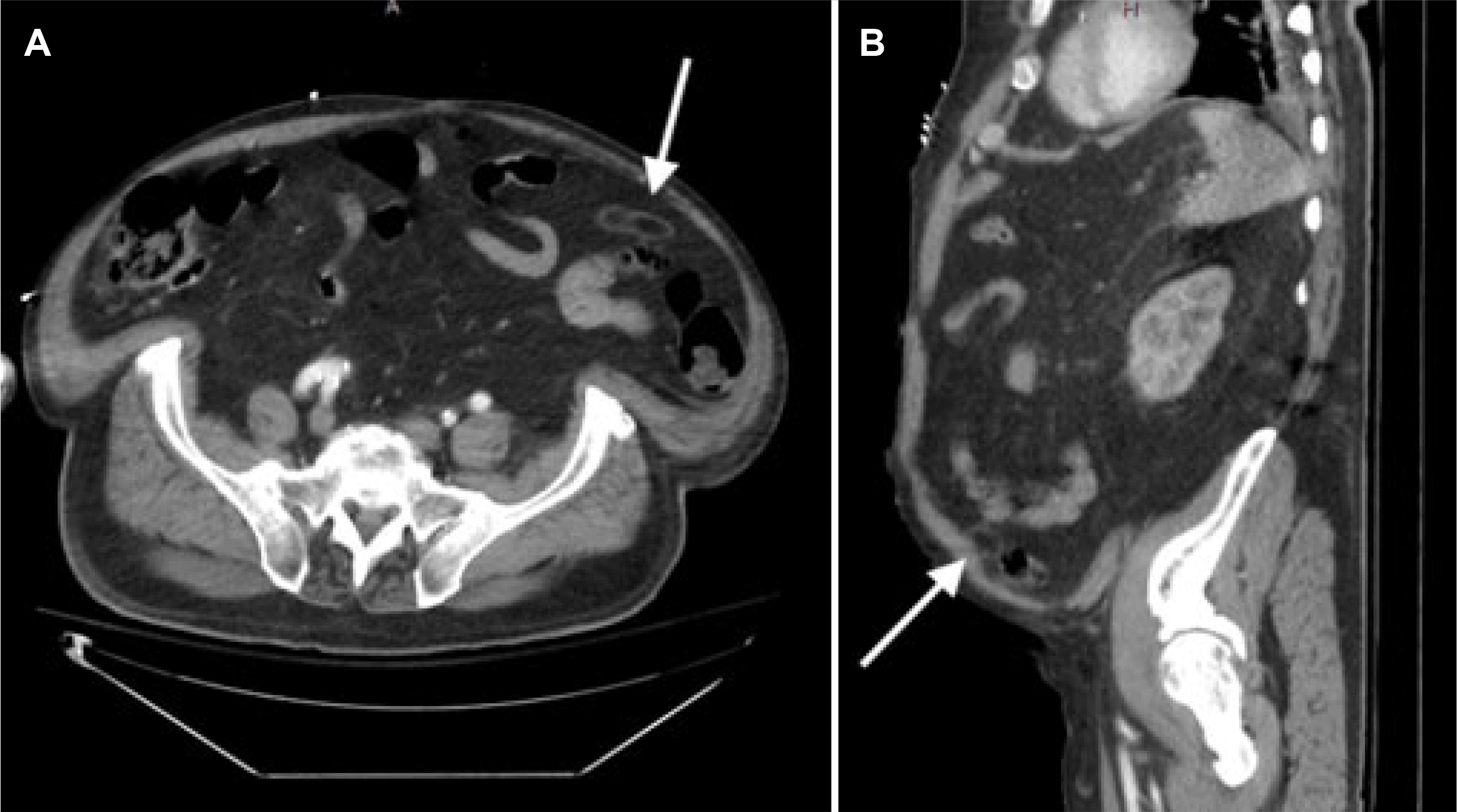
General surgery was considered because of cholelithiasis and pain out of proportion to the radiographic findings. A hepatobiliary iminodiacetic acid scan showed no biliary obstructions. Despite being treated empirically for severe sepsis, the patient experienced worsening abdominal pain and worsening hypoxic hypercapnic respiratory failure, ultimately requiring intubation. A repeat CT scan of the abdomen and pelvis with contrast was performed because of the patient’s rapid and progressive clinical decline, with the following new finding: small focus of fat necrosis adjacent to the distal descending and sigmoid colon possibly secondary to a torsed epiploic appendage/EA (Fig. 2).
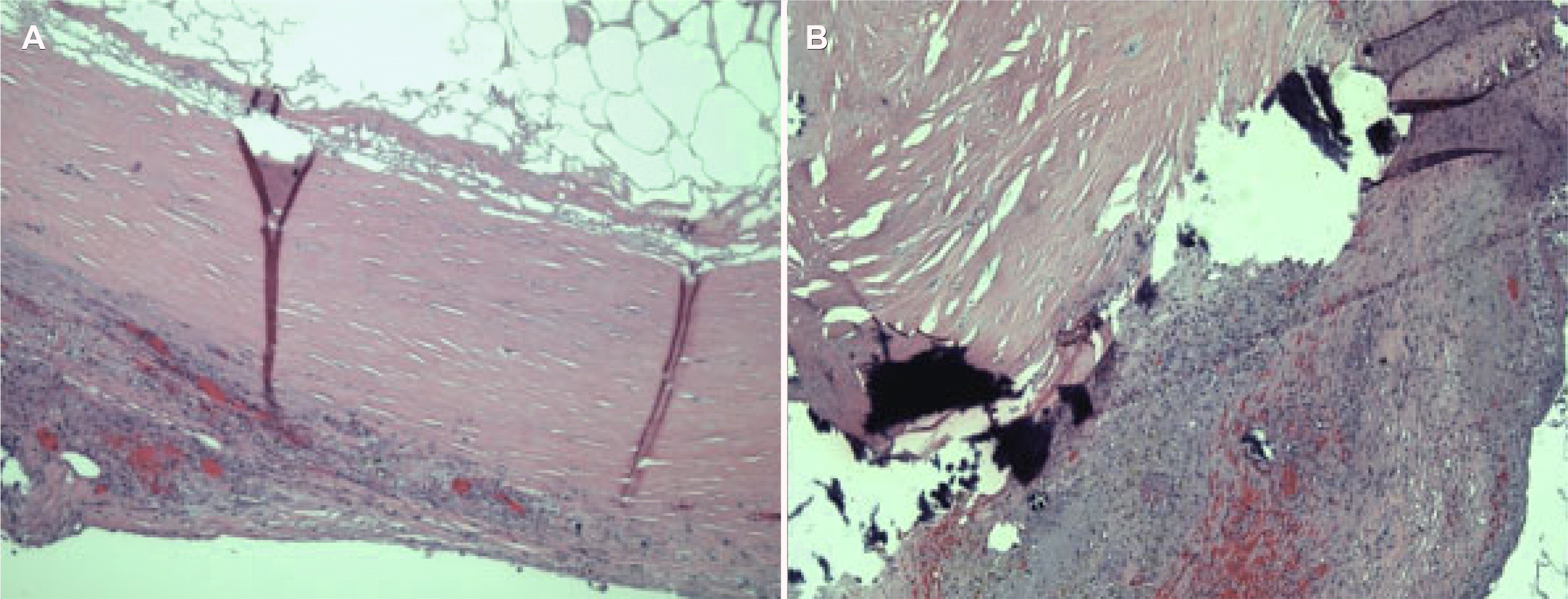
Considering the patient’s worsening clinical status, an exploratory laparoscopy was performed. A Veress needle was placed in the left upper quadrant at the Palmer’s point, and the abdominal cavity was insufflated to 15 mmHg. A 7 mm incision was made in the left upper quadrant with the placement of a 5 mm trocar. The Veress needle was removed, and two additional 5 mm trocars were placed on the left side of the abdomen. The small bowel was then run from the ileocecal valve back to the ligament of Treitz without evidence of ischemic bowel or bowel swelling. Minimal ascites were identified in the abdominal cavity. A colon examination showed no evidence of appendicitis but findings of an inflamed epiploic appendage near the descending and sigmoid colon juncture. The appendage was excised laparoscopically and removed. Postoperatively, the patient’s respiratory status improved rapidly, and he was extubated the following morning. The patient was downgraded from the ICU two days postoperatively, and he completed seven days of total antibiotic therapy in the hospital. He was then discharged to a rehabilitation facility and recovered without complications or a new onset of abdominal pain. The pathology report described the gross specimen as white–tan and ovoid, measuring 5×2.7×1.3 cm in size. The specimen was previously sectioned and completely opened to reveal a cystic lesion with a wall measuring up to 0.1 cm in thickness, filled with a yellow fatty content, and a 5×3.5×0.8 cm segment of fibroadipose tissue was attached to the cystic lesion. The microscopic diagnosis was fibrous cystic wall-like tissue with chronic inflammation and dystrophic calcification (Fig. 3).
Epiploic appendages are a normal anatomical feature, consisting of approximately 50–100 fatty outpouchings distributed axially from the cecum to the rectosigmoid, with approximately 57% located in the sigmoid colon and 26% in the cecum.10 On the other hand, ischemia of these outpouchings produces EA, commonly resulting in abrupt focal, non-migratory abdominal pain and tenderness to palpation without nausea or vomiting and with normal vital signs and laboratory results.8 A slight elevation in white blood cell count and C-reactive protein may be observed because of the inflammatory response induced by ischemic fat necrosis.2 Considering the relative rarity of EA as the etiology of abdominal pain at 1.1–1.3% of all cases, EA is frequently misdiagnosed as several alternative pathologies, with the most frequent misdiagnoses being acute uncomplicated diverticulitis and acute appendicitis.11,12 Clinically differentiating between EA and acute uncomplicated diverticulitis can be difficult due to overlap of the presenting symptoms. Both share focal, constant abdominal pain, most often in the left lower quadrant, rarity of nausea and vomiting, and a frequently normal white blood cell count.13 Some clinical differentiators of diverticulitis versus EA include changes in bowel habits, such as constipation and diarrhea (50% and 25–35% of diverticulitis cases, respectively), fever, and a commonly elevated C-reactive protein.14,15 In definitively differentiating acute uncomplicated diverticulitis from EA, a computed tomography scan yields a sensitivity and specificity for diagnosing diverticulitis of 94 and 99%, respectively.16 Such imaging in diverticulitis will often demonstrate localized bowel wall thickening and increased soft tissue density (fat stranding) within the pericolonic fat because of inflammation. EA classically manifests as a 1.5–3.5 cm sized ovoid mass in the maximal diameter with fat attenuation, surrounded by a hyperdense ring corresponding to the inflammatory reaction of the overlying serosa.5,17 In contrast to many alternative etiologies of an acute abdomen, EA is typically self-limited and managed conservatively with analgesics.6
As outlined previously, EA does not produce systemic signs, underscoring the uniqueness of this case. This paper reports the first case of EA that produced sepsis and acute respiratory failure. While causation could not be definitively determined from an observational case, the very close correlation between this patient’s rapid recovery immediately following an epiploic appendagectomy gives compelling support for EA as the source of his critical condition. A thorough review of the literature did not yield a single case of EA producing such dramatic systemic symptoms. EA is frequently cited as an important differential to consider during the workup of an acute abdomen. Therefore, EA should be considered as a rare source of decline in the critically ill.
Notes
Financial support
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
REFERENCES
1. Nemoto H, Yoshizawa Y, Hibi K, et al. 2008; Strangulation caused by a small bowel epiploic appendage: report of a case. Case Rep Gastroenterol. 2:214–218. DOI: 10.1159/000135609. PMID: 21490891. PMCID: PMC3075146.
2. Sand M, Gelos M, Bechara FG, et al. 2007; Epiploic appendagitis--clinical characteristics of an uncommon surgical diagnosis. BMC Surg. 7:11. DOI: 10.1186/1471-2482-7-11. PMID: 17603914. PMCID: PMC1925058.
3. Giambelluca D, Cannella R, Caruana G, et al. 2019; CT imaging findings of epiploic appendagitis: an unusual cause of abdominal pain. Insights Imaging. 10:26. DOI: 10.1186/s13244-019-0715-9. PMID: 30796645. PMCID: PMC6386757.
4. Ghahremani GG, White EM, Hoff FL, Gore RM, Miller JW, Christ ML. 1992; Appendices epiploicae of the colon: radiologic and pathologic features. Radiographics. 12:59–77. DOI: 10.1148/radiographics.12.1.1734482. PMID: 1734482.
5. Giannis D, Matenoglou E, Sidiropoulou MS, et al. 2019; Epiploic appendagitis: pathogenesis, clinical findings and imaging clues of a misdiagnosed mimicker. Ann Transl Med. 7:814. DOI: 10.21037/atm.2019.12.74. PMID: 32042830. PMCID: PMC6989878.
6. Macari M, Laks S, Hajdu C, Babb J. 2008; Caecal epiploic appendagitis: an unlikely occurrence. Clin Radiol. 63:895–900. DOI: 10.1016/j.crad.2007.12.016. PMID: 18625354.
7. Di Serafino M, Iacobellis F, Trovato P, et al. 2019; Acute epiploic appendagitis: A nonsurgical abdominal pain. Case Rep Emerg Med. 2019:7160247. DOI: 10.1155/2019/7160247. PMID: 31380126. PMCID: PMC6662477.
8. Rioux M, Langis P. 1994; Primary epiploic appendagitis: clinical, US, and CT findings in 14 cases. Radiology. 191:523–526. DOI: 10.1148/radiology.191.2.8153333. PMID: 8153333.
9. Patel VG, Rao A, Williams R, Srinivasan R, Fortson JK, Weaver WL. 2007; Cecal epiploic appendagitis: a diagnostic and therapeutic dilemma. Am Surg. 73:828–830. DOI: 10.1177/000313480707300821. PMID: 17879696.
10. Gourgiotis S, Oikonomou C, Veloudis G, Lardou I, Pittaras G, Villias C. 2016; The diagnostic dilemma of primary epiploic appendagitis and how to establish a diagnosis. Oman Med J. 31:235–237. DOI: 10.5001/omj.2016.45. PMID: 27162597. PMCID: PMC4852085.
11. Garg R, Ma D, Fishbain JT. 2018; Epiploic appendagitis: The uncommon intestinal imitator. Clin Gastroenterol Hepatol. 16:A36. DOI: 10.1016/j.cgh.2017.05.034. PMID: 28554681.
12. Kefala MA, Tepelenis K, Stefanou CK, et al. 2020; Primary epiploic appendagitis mimicking acute appendicitis: A case report and narrative review of the literature. Korean J Gastroenterol. 76:88–93. DOI: 10.4166/kjg.2020.76.2.88. PMID: 32839372.
13. Ambrosetti P, Robert JH, Witzig JA, et al. 1994; Acute left colonic diverticulitis: a prospective analysis of 226 consecutive cases. Surgery. 115:546–550.
14. Konvolinka CW. 1994; Acute diverticulitis under age forty. Am J Surg. 167:562–565. DOI: 10.1016/0002-9610(94)90098-1. PMID: 8209928.
15. Bailey J, Dattani S, Jennings A. 2022; Diverticular disease: Rapid evidence review. Am Fam Physician. 106:150–156.
16. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. 2008; Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 18:2498–2511. DOI: 10.1007/s00330-008-1018-6. PMID: 18523784.
17. Birnbaum BA, Balthazar EJ. 1994; CT of appendicitis and diverticulitis. Radiol Clin North Am. 32:885–898.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download