1. Standring S. Standring S, editor. 2008. Mediastinum. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 40th ed. Churchill Livingstone;p. 939–57. DOI:
10.1016/b978-0-443-06684-9.50063-9.
2. Snell RS. 2004. Clinical Anatomy. Lippincott Williams & Wilkins.
3. Drake RL, Vogl W, Mitchell AWM. 2015. Gray's Anatomy for Students. 3rd ed. Elsevier;p. 197.
4. Celik HH, Sargon MF, Aldur MM, Cumhur M. 1996; An anomalous course of the interazygos vein. Surg Radiol Anat. 18:61–2. DOI:
10.1007/BF03207766. PMID:
8685815.

5. Mezzogiorno A, Passiatore C. 1988; An atypic pattern of the azygos venous system in man. Anat Anz. 165:277–81. DOI:
10.53347/rid-26571. PMID:
3400891.
6. Casoni GL, Tomassetti S, Cavazza A, Colby TV, Dubini A, Ryu JH, Carretta E, Tantalocco P, Piciucchi S, Ravaglia C, Gurioli C, Romagnoli M, Gurioli C, Chilosi M, Poletti V. 2014; Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 9:e86716. DOI:
10.1371/journal.pone.0086716. PMID:
24586252. PMCID:
PMC3938401. PMID:
79250b8f3a59492884f75f26253863fa.

7. Seib GA. 1934; The azygos system of veins in American whites and American negroes, including observations on the inferior caval venous system. Am J Phys Anthropol. 19:39–163. DOI:
10.1002/ajpa.1330190117.

8. Rosse C, Gaddum-Rosse P. 1997. Hollinshead's Textbook of Anatomy. 5th ed. Lippincott-Raven.
9. Shivanal U, HT G. 2015; Anomalous azygos veins - its embryological basis and clinical significance. Int J Res Med Sci. 3:2323–6. DOI:
10.18203/2320-6012.ijrms20150624.

10. Piciucchi S, Barone D, Sanna S, Dubini A, Goodman LR, Oboldi D, Bertocco M, Ciccotosto C, Gavelli G, Carloni A, Poletti V. 2014; The azygos vein pathway: an overview from anatomical variations to pathological changes. Insights Imaging. 5:619–28. DOI:
10.1007/s13244-014-0351-3. PMID:
25171956. PMCID:
PMC4195836.

11. Romanes GJ. Romanes GJ, editor. 1986. The thorax. Cunningham's Manual of Practical Anatomy, Vol 2: Thorax and Abdomen. 15th ed. Oxford University Press;p. 1–82. DOI:
10.5005/jp/books/14241_6.
12. Paraskevas GK, Koutsouflianiotis KN, Patsikas M, Noussios G. 2019; What is the history of the term "azygos vein" in the anatomical terminology? Surg Radiol Anat. 41:1155–62. DOI:
10.1007/s00276-019-02238-3. PMID:
31028449.

13. Dubois J. 1555. In Hippocratis et Galeni Physiologiae Partem Anatomicam Isagoge. Apud Joannem Hulpeau;p. 18924. Latin.
14. Vesalius A. 1543. De Humani Corporis Fabrica Libri Septem. Ex Officina Joannis Oporini;p. 37. Latin. DOI:
10.5962/bhl.title.109299.
15. Prasad M, K J. 2018; Variations in formation and termination of azygos vein in South Indian population. Int J Anat Res. 6:5384–9. DOI:
10.16965/ijar.2018.212.

16. Radhika D, Nirmala BV, Latha GK. 2016; Tributaries of azygos vein. J Evid Based Med Healthc. 3:49–51. DOI:
10.18410/jebmh/2016/11. PMID:
27871101.

17. Nirmala BV, Teresa RS. 2015; Study of azygos system and its variations. J Evol Med Dent Sci. 4:5652–7. DOI:
10.14260/jemds/2015/827.

18. Latha K, Sugavasi R. 2013; Study of the azygos system of veins in human cadaver. Int J Curr Res Rev. 5:113–7.
19. Alves EC, Porciúncula Junior RW, Monte Bispo RF, de Sousa-Rodrigues CF, da Rocha AC. 2011; Formation of the azygos vein. Int J Morphol. 29:140–3. DOI:
10.4067/S0717-95022011000100024.
20. Mustafa SIA, Ali TO, Ahmed AA. 2016; Anatomical variation of the azygos vein in human cadavers. Int J Innov Sci Res. 25:368–72.
21. Krakowiak-Sarnowska E, Wiśniewski M, Szpinda M, Krakowiak H. 2003; Variability of the azygos vein system in human foetuses. Folia Morphol (Warsz). 62:427–30. PMID:
14655133.
22. Vedapriya KA, Priyanka D. 2019; Morphological variation in azygos vein. Int J Sci Res. 8:1396–9. DOI:
10.32388/rqli8f.
24. Saito A, Murakami M, Tomioka K, Ezure H, Moriyama H, Mori R, Nakajima K, Nakamura M, Otsuka N. 2015; The impact of aging on the course of the azygos vein. Okajimas Folia Anat Jpn. 92:7–10. DOI:
10.2535/ofaj.92.7. PMID:
26448373.

26. Tatar I, Denk CC, Celik HH, Oto A, Karaosmanoglu DA, Ozdemir BM, Surucu SH. 2008; Anatomy of the azygos vein examined by computerized tomography imaging. Saudi Med J. 29:1585–8. DOI:
10.53347/rid-955. PMID:
18998005.
27. Raghavendra AY, Bhosale SM. 2017; Variations of arch of azygos vein: an anatomical overview with clinical importance. Int J Anat Res. 5:4251–6. DOI:
10.16965/ijar.2017.299.

28. Kutoglu T, Turut M, Kocabiyik N, Ozan H, Yildirim M. 2012; Anatomical analysis of azygos vein system in human cadavers. Rom J Morphol Embryol. 53:1051–6. PMID:
23303031.
29. Patra A, Singla RK, Kaur H, Malhotra V. 2019; Analysis of multiple variations in azygos venous system anatomy with its classification: a cadaveric study. Eur J Anat. 23:9–15.
30. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. 2000; Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 20:639–52. DOI:
10.1148/radiographics.20.3.g00ma09639. PMID:
10835118.

31. Milne EN. 1978; Some new concepts of pulmonary blood flow and volume. Radiol Clin North Am. 16:515–36. PMID:
746145.
34. Preger L, Hooper TI, Steinbach HL, Hoffman JI. 1969; Width of azygos vein related to central venous pressure. Radiology. 93:521–3. DOI:
10.1148/93.3.521. PMID:
4898452.

35. Seema MA. 2012; A rare anatomical variation in medial root of azygos vein with its embryological aspect. Int J Anat Sci. 3:14–6.
36. Dahran N, Soames R. 2016; Anatomical variationos of the azygos venous system: classification and clinical relevance. Int J Morphol. 34:1128–36. DOI:
10.4067/S0717-95022016000300051.
37. Rao YS, Banerjee A. 2014; Anatomical variations of the azygos venous system. Int J Biol Med Res. 5:4535–8. DOI:
10.1142/9781783269150_0048.
39. Moore KL, Persaud TVN, Torchia MG. Torchia MG, Persaud TVN, editors. 2016. Cardiovascular system. The Developing Human: Clinically Oriented Embryology. 10th ed. Elsevier;p. 283–334.
40. Kc S, Shrestha P, Shah AK, Jha AK. 2018; Variations in human pulmonary fissures and lobes: a study conducted in Nepalese cadavers. Anat Cell Biol. 51:85–92. DOI:
10.5115/acb.2018.51.2.85. PMID:
29984052. PMCID:
PMC6026823.

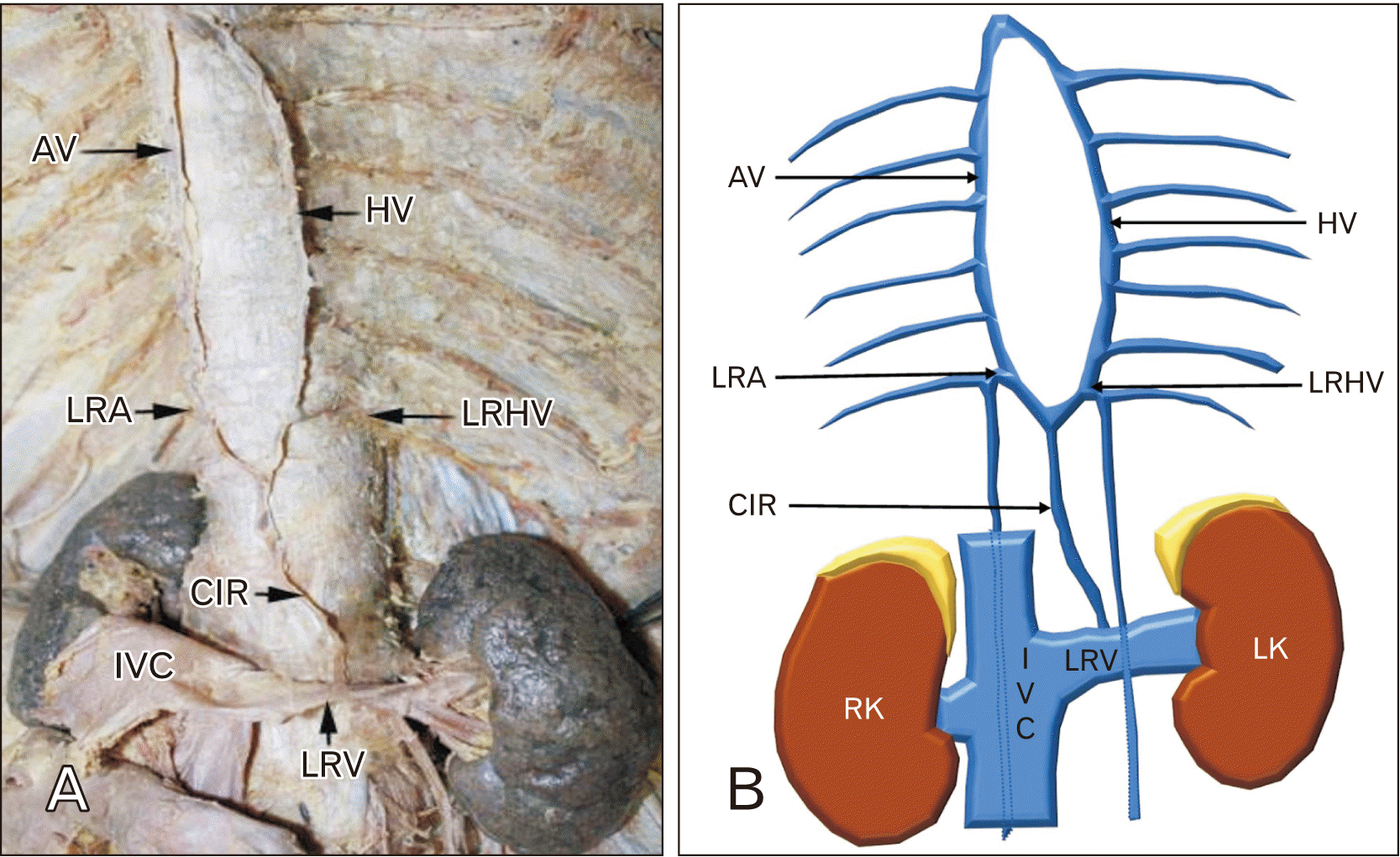




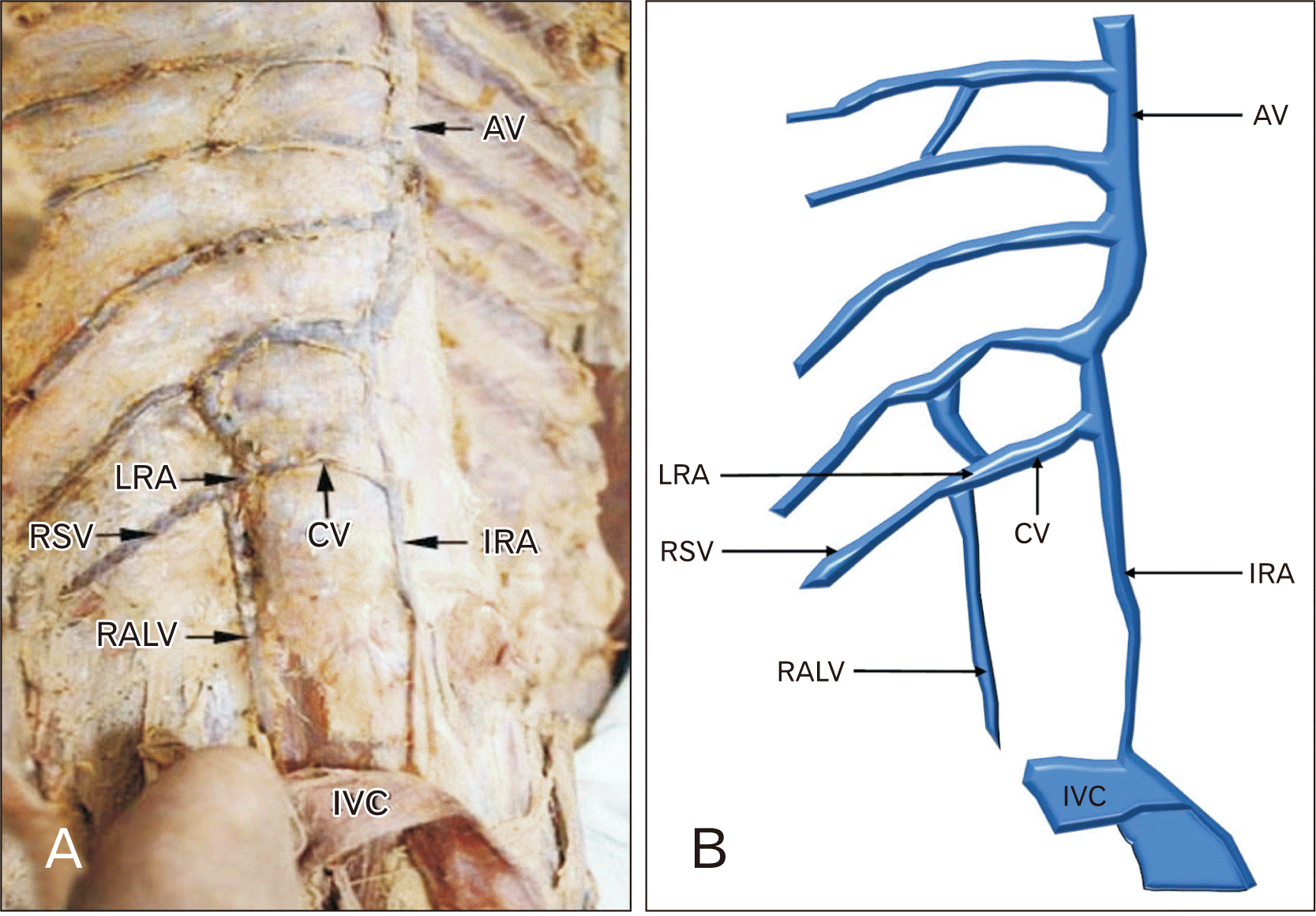
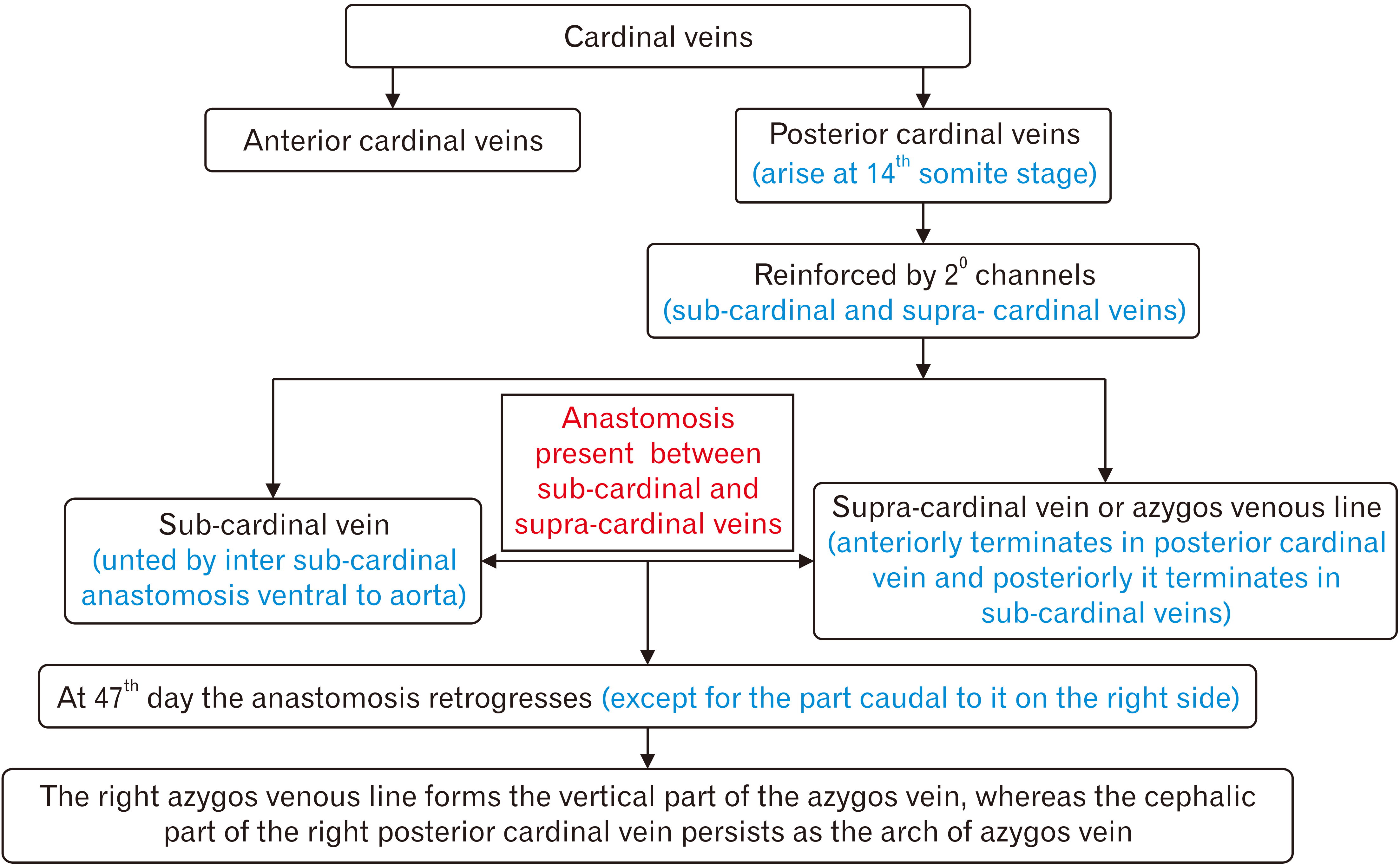
 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download