Introduction
The digastric muscle is an essential landmark in the area of the neck. It belongs to the suprahyoid group of muscles. The primary function of the suprahyoid group is depression of the mandible and elevation and fixation of the hyoid bone [
1]. The submental group of lymph nodes that drains the oral cavity’s midline structures and tongue tip lies between two anterior bellies of the digastric muscle [
2]. The submandibular region lies between the anterior and posterior bellies of the digastric muscle
[
3]. The chief structures in this area are the submandibular gland, facial vessels, marginal mandibular nerve, lymph nodes, hypoglossal nerve, and carotid sheath with its contents [
4].
The digastric muscle is known to have shown many variations mainly because the embryological origin of this muscle is from two pharyngeal arches [
5]. The anterior belly is from the first arch, and the nerve supplying it is a branch from the mandibular nerve, the nerve of the first arch. The posterior belly originates from the second arch, and the nerve supplying it is a branch from the facial nerve, which is the nerve of the second arch [
6]. A computerized morphometrical analysis study done previously on 74 cadavers classified the anterior belly of digastric into five types, the intermediate tendon into three types, and the posterior belly into two kinds. Subsequently, combinations of these types have been grouped into unique patterns named from A to J [
7]. Such observations aid in interpreting morphological variation and correlating them with clinical observations.
An accessory anterior belly of digastric is not uncommon in the literature [
3,
4,
7,
8]. Accessory muscle slips, when present, may sometimes cause pharyngeal pain and foreign body sensations in the throat. These extra slips may also give variant mobility to the hyoid bone leading to symptoms similar to the stylohyoid syndrome. Also, the anterior belly of the digastric is removed in platysmarrhaphy and is effectively utilized for reconstructive surgeries after the palsy of the marginal mandibular branch of the facial nerve [
9,
10].
Although the variation of the digastric muscle does not display any noticeable symptoms, surgical advances in maxillofacial, reconstructive, and aesthetic surgery make it essential for surgeons to know the morphology of the muscle. In cosmetic neck surgeries to achieve a well-contoured submental region, partial resection of the large bulky anterior belly of the digastric muscle is performed [
8]. Further, the supernumerary and accessory bellies of the muscle should also be looked for and removed by the surgeon for the best result of these procedures. Since the digastric muscle is a significant surgical landmark in neck dissections, morphometric measurements of this muscle thus become imperative. The major bony landmarks surrounding the digastric muscle should also be considered during the morphometric measurements. It would further be useful in establishing the correlation between muscle lengths and the neck.
Knowledge of the morphometry of the digastric muscle is essential to avoid misdiagnosis when evaluating abnormal swellings in the submental region [
11]. Previous studies have concentrated on the unusual variation of the digastric muscle. However, the existing literature is deficient in the knowledge of morphometric data of the muscle. Hence, this study aims to provide adequate data to help clinicians in the therapeutic and surgical interventions in the area.
Materials and Methods
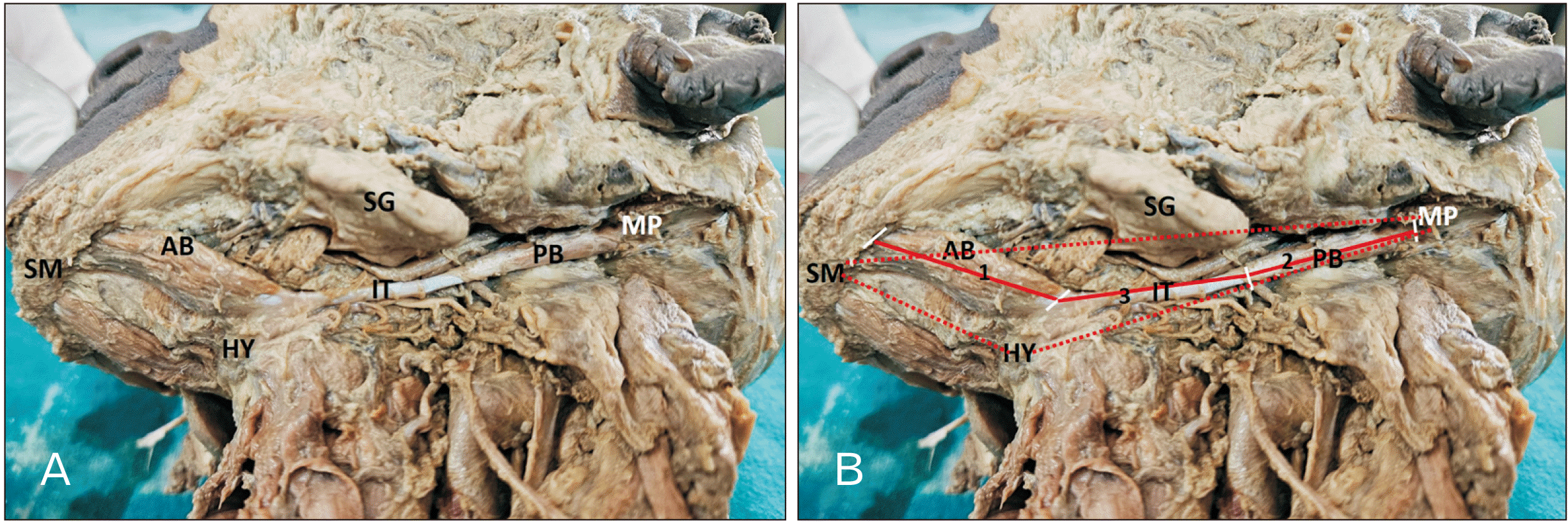
The current cross-sectional evaluative study involved forty-two human cadaveric specimens (27 males and 15 females). All the intact cadaveric specimens of the neck were included in the study. Two cadavers with variation in the digastric muscle (i.e., accessory bellies) were excluded from morphometric measurements. The neck was dissected by giving a midline incision from the symphysis menti to the jugular notch, and the skin was reflected inferiorly and laterally. The platysma was reflected superiorly. The sternocleidomastoid was cleared off the fat and fascia from its superficial surface and margins. The intermediate tendon of the digastric was identified. The attachment of the anterior belly of the digastric to the mandible was noted, and the distal attachment of the posterior belly to the mastoid notch was traced. The measurements were taken using a thread and scale. The measurements were recorded with the cadaveric jaw closed.
The following measurements were considered:
Length of the anterior belly of digastric (from the digastric fossa of the mandible to its intermediate tendon, i.e., as soon as the muscle becomes tendon)
Length of the posterior belly of digastric (from the intermediate tendon to mastoid notch attachment)
Length of the intermediate tendon (the point as soon as the anterior muscle belly becomes tendon and as soon as the tendon ends and posterior belly begins).
Additionally, the following anthropometric measurements were taken (as standard bony reference points to compare the measurements of the digastric muscle):
Distance from symphysis menti to hyoid bone
Distance from hyoid bone to mastoid process
Distance from symphysis menti to mastoid process.
Ethical statement
The present study was conducted on cadavers donated (with legally acceptable relative [LAR] consent) for medical education and research. Only Departmental (Scientific) Review Committee approval was obtained as cadaver-based studies were exempt from submission to ethics committee during the time frame of conduct of research (2016-17).
Statistical analysis
GraphPad Prism Software (version 6) was used to analyze the morphometric measurements statistically. The paired t-test was applied to find differences between the right and left sides. An independent t-test was applied to detect differences between male and female sizes. Pearson’s correlation coefficient was determined to find a correlation between the length of the neck and the length of the digastric muscle. A scatter plot graph was used to plot the same.
Results
Forty-two cadavers were involved in the present study. However, the morphology and morphometry of the digastric muscle were studied in 40 cadavers (25 males and 15 females), in which the normal anatomy of the anterior and posterior bellies of the digastric with its intermediate tendon was observed (
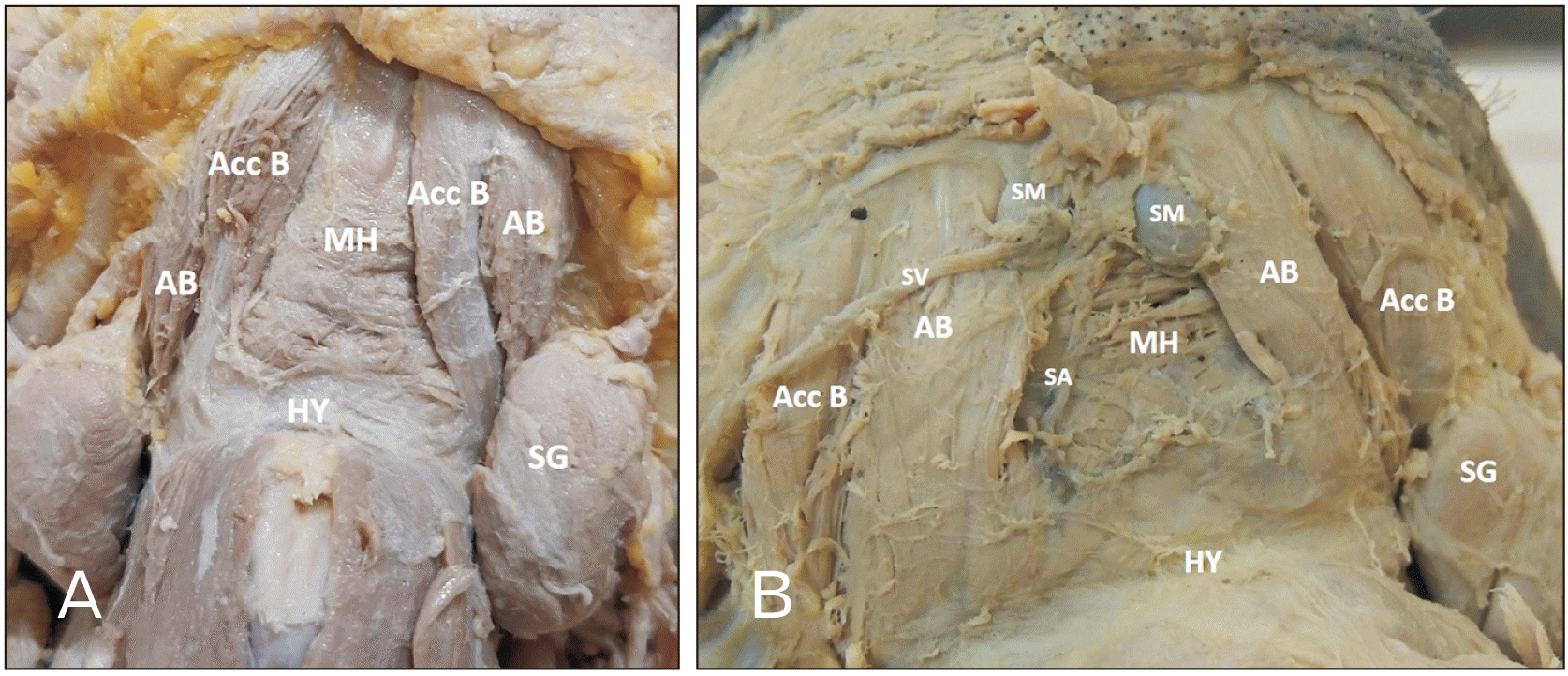
Fig. 1). The two cadavers which showed morphological variations were not considered for the morphometric observations (
Fig. 2).
Morphometric observations
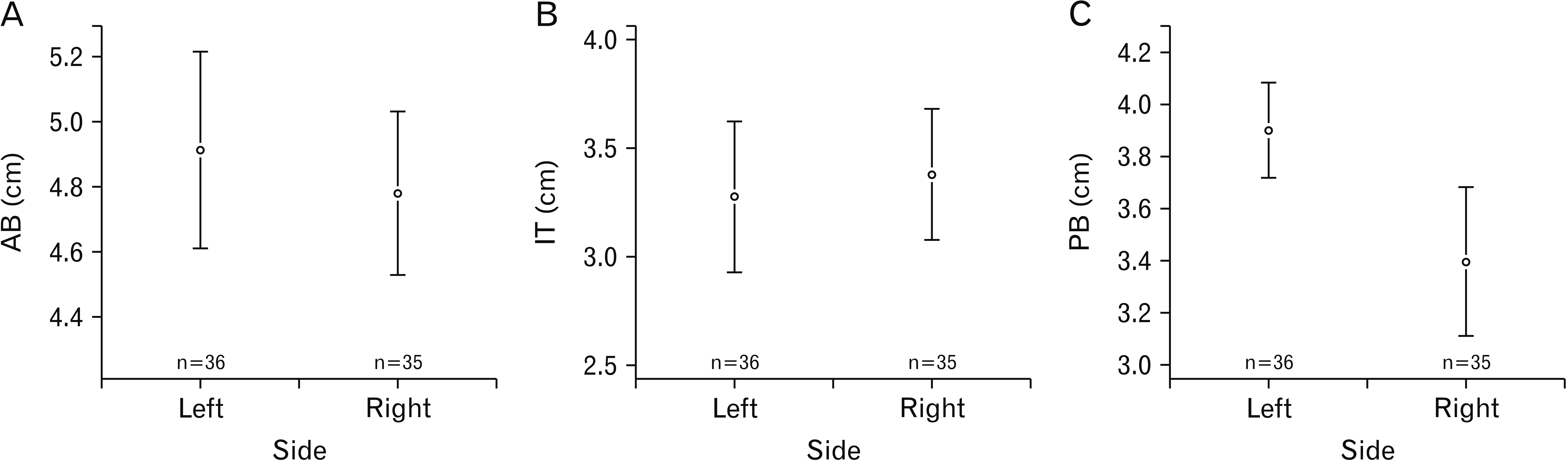
The morphometric observations are represented in
Table 1. The dimensions (in length) of the anterior belly and intermediate tendons of the right and left showed no significant difference statistically. However, a significant difference was appreciated between the right and left sides of the posterior belly (
P=0.003) (
Fig. 3). A statistically significant difference between males and females was seen in the length of the anterior belly (
P=0.04) as well as of the intermediate tendon (
P=0.04). But the posterior belly did not show any noteworthy difference in length between genders (
Table 1).
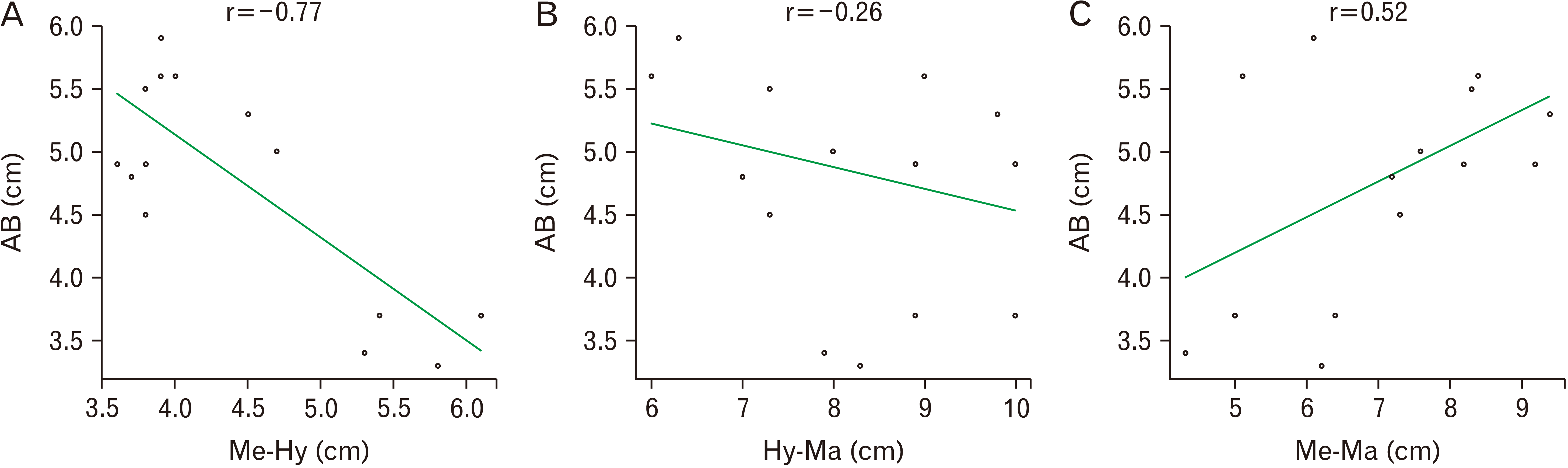
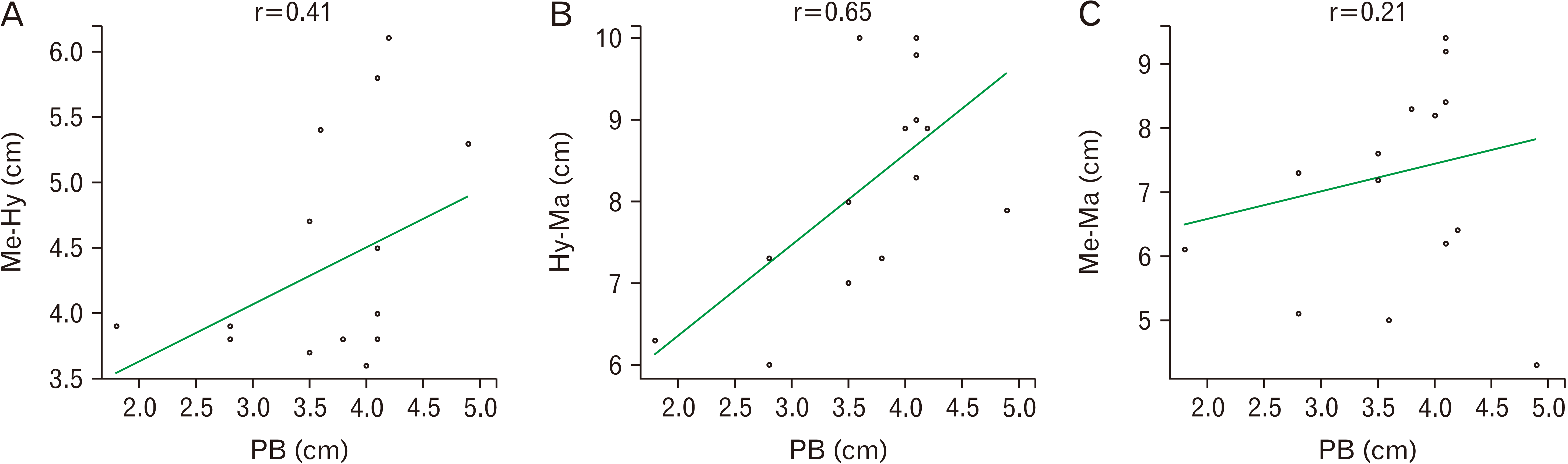
Further, the length of the neck measured between bony landmarks of the hyoid, symphysis menti, and mastoid process, when correlated with the dimensions of the digastric, exhibited a negative linear relationship with the anterior belly,
i.e., r=–0.77, –0.26, and 0.52 (
Fig. 4). On the contrary, a positive linear relationship was noted with the posterior belly,
i.e., r=0.41, 0.65, and 0.2 (
Fig. 5).
Anatomical variation
In the study, two cadavers showed the presence of bilateral accessory bellies of the anterior belly of the digastric (
Fig. 2).
Case 1: In a 56-year-old male cadaver, the accessory bellies on both sides originated from the medial side of the anterior bellies in the digastric fossa. The muscle fibers of the accessory belly were directed downwards, backward, and laterally. It was inserted into the lateral part of hyoid bone on both sides (
Fig. 2A).
Case 2: In a 45-year-old male cadaver, the accessory bellies on both sides originated from the lateral side of the anterior bellies in the digastric fossa. The muscle fibers of the accessory belly were directed downwards, backward, and laterally. It was inserted into the intermediate tendon on both sides (
Fig. 2B).
In both cases, the thickness of the accessory bellies was equal to that of the anterior bellies of the digastric. The innervation to these accessory muscles was a branch from the nerve supplying the anterior belly of the digastric on both sides. The accessory muscle bellies were identical on the right and left side concerning size, shape, origin, insertion, and nerve supply.
Discussion
Variances of the anterior bellies of the digastric muscles are infrequent, but lately, radiologists and maxillofacial surgeons have drawn attention to their occurrence to evade confusion with anomalous lesions when scanning the floor of the mouth and the submental space
[
12].
According to a morphometric study by Zdilla et al. [
13] on 35 specimens, males had longer left-sided muscle bellies. No difference was noted between the length of the muscle bellies and the intermediate tendon of the right and left sides. They found no relationship between the length of the belly and the intermediate tendon [
13]. In this study, the males had longer anterior bellies and intermediate tendons compared to the females, and the right-sided posterior belly was longer than the left side.
According to De-Ary-Pires et al. [
7], the classification of the digastric muscle involves unique patterns from A to J. Our specimens were mostly in pattern A, which is the most common variety and is 66% prevalent in the previous study. In the present study, a negative linear correlation was found between the anterior belly and the length of the neck signifying that as the morphometry of the neck increases the anterior belly length decreases. However, it showed a positive correlation with the posterior belly suggesting that the length of the posterior belly increases with an increase in the length of the neck.
In previous studies, ultrasound and magnetic resonance imaging (MRI) were used to measure the cross-sectional area of the digastric muscle. It was found that ultrasound measurements were smaller than MRI measurements by 10%, but the correlations were significant and high, concluding that ultrasound is a viable imaging technique [
14,
15].
A variation of the right and left anterior bellies connected to each other at their insertion on the hyoid bone was found by Kalniev et al. [
16]. In addition, a rare variation of four bellies of the anterior digastric muscles bilaterally with variants of the median accessory digastric muscles was found by Ozgur et al. [
17]. Two accessory bellies, medial to the two normal anterior bellies of the digastric muscle merge and attach at the mylohyoid raphe were reported by Kyung et al. [
18]. A case of three accessory bellies of digastric muscle with one fibrous band was also found to be attached to the mylohyoid raphe [
19].
In a case reported by Singh et al. [
20] the accessory anterior belly of the digastric muscle was present bilaterally. The anterior belly was absent on the right side. The accessory belly was superficial to the mylohyoid raphe on the right side and to the regular digastric muscle on the left side.
Yamazaki et al. [
21] stated an occasion of four additional anterior bellies cases, a slender accessory anterior belly, which has its natural origin, gets inserted into the second anterior accessory belly. It was observed that the right and left accessory anterior belly originating from the mylohyoid raphe inserted into the intermediate tendon, connecting the left intermediate tendon to the right intermediate tendon, a transverse accessory anterior belly.
Holibková et al. [
22] noted the presence of bilateral accessory bellies as the medial and lateral head, the latter of which passed through the intermediate tendon and fixed partially on the hyoid bone. In another case, the asymmetrical anomaly of the anterior belly was observed. The anterior belly bundle from the left side was inserted into the intermediate tendon of the right side and the hyoid bone.
A variation case with bilateral accessory anterior digastric muscle was reported by Peker et al. [
23] in which the right anterior belly origin was from mylohyoid raphe and inserted on the intermediate tendon of the right side. Similarly, originating from the mylohyoid raphe, the left accessory belly was inserted on the left intermediate tendon. In the present study, the accessory anterior bellies were bilateral in both cases. In case 1, the accessory bellies were arising medial to the anterior belly and inserted into the hyoid bone, while in case 2, the accessory bellies were arising lateral to the anterior bellies and inserted into the intermediate tendon.
The existence of such variations can affect the radiological interpretation of enlarged lymph nodes in the area. Metastasis to the cervical lymph nodes usually occurs in carcinoma of the oral cavity. Hence muscular variations could be mistaken for anomalous masses [
10,
13,
15].
Digastric muscle transplant is used in corrective surgery for lower lip asymmetry and harvested in a pedicled submental flap. Further, the digastric muscle sew-up procedure for the repair of the floor of mouth following pull-through operations for advanced oral cancers has also been found to be simple, safe, and time-saving for small to medium-sized defects of the floor of mouth created by ablative surgery. Accessory muscle slips when present can provide supplementary tissue to work within these cases [
24,
25]. Morphometric measurements related to the muscle may also aid in this process.
Surgical advances in maxillofacial, reconstructive, and aesthetic surgery make it essential for surgeons to understand the anatomical features of the digastric muscle. Morphometric measurements of the digastric muscle thus become imperative. Also, the supernumerary and accessory bellies of the muscle should also be looked for and removed by the surgeon for the best result of these procedures. Knowledge of the morphology and morphometry of the digastric muscle is also essential to avoid misdiagnosis when evaluating abnormal swellings in the submental region [
11]. Recognizing the digastric muscle variations which can be misinterpreted as metastasized lymph nodes or tumor masses can help the surgeons avoid unnecessary surgeries in the neck [
26].
Limitations of the study
The sample size i.e., the number of specimens would reinforce a clear picture if it were larger. A review of the percentage of variations existing in the digastric muscle and its possible clinical implications could also be attempted and tabulated (in the present study there were only two incidental findings as described in cases 1 and 2). Further, the cadaveric data could be compared with the ultrasound findings for better validation. These aspects can be considered by anatomists interested in furthering the research in this area.
The morphology as well as the morphometry of the digastric muscle is vital for clinicians and maxillofacial surgeons while dealing with the pathology of the submental and submandibular region. This study provides and co-relates normal morphometric data of the muscle to the various bony landmarks of the neck.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download