Abstract
Minimally invasive pancreatoduodenectomy (MIS PD) is a well reported technique with several advantages over conventional open pancreatoduodenectomy. In comparison to distal pancreatectomy, the adoption of MIS PD has been slow due to the technical challenges involved, particularly in the reconstruction phase of the pancreatojejunostomy (PJ) anastomosis. Hence, we introduce a low-cost model for PJ anastomosis simulation in MIS PD. We fashioned a model of a cut pancreas and limb of jejunum using economical and easily accessible materials comprising felt fabric and the modelling compound, Play-Doh. Surgeons can practice MIS PJ suturing using this model to help mount their individual learning curve for PJ creation. Our video demonstrates that this model can be utilized in simulation practice mimicking steps during live surgery. Our model is a cost-effective and easily replicable tool for surgeons looking to simulate MIS PJ creation in preparation for MIS PD.
Surgery remains the mainstay of treatment for resectable pancreatic adenocarcinoma. Over the years, pancreatoduodenectomy (PD) for pancreatic head and uncinate process is considered one of the most technically challenging abdominal surgical operations. Challenges in this operation include the retroperitoneal location of pancreas, and proximity to major vascular structures, as well as the number of anastomoses required for functional reconstruction [1]. As the adoption of minimally invasive (MIS) increases worldwide, experienced surgeons have pushed the boundaries and extended the role of MIS from left-sided pancreas resections to include PD. Meta-analytic comparisons of MIS PD with conventional open surgery have demonstrated encouraging outcomes of improved blood loss, and similar post-operative and oncologic outcomes, albeit longer operative times [2].
The slower adoption of MIS PD and longer operative times are largely contributed by the technical challenges involved, in particular the pancreatojejunostomy (PJ) anastomosis. PJ anastomosis requires meticulous and precise suturing skills, often at taxing angles and depths. In this article, we describe a low-cost and easily reproducible model for surgeons to simulate MIS PJ anastomosis.
Our model comprises low-cost materials of commercially available handicraft felt that is a textile produced by condensed and matted fibers, Play-Doh, and sutures. The felt fabric is chosen, as it has appropriate thickness, and compressible consistency that mimics small bowel and pancreatic capsule. Play-Doh is a commonly available modelling compound made from a mixture of water, salt, and flour, together with other additives, which is used to create a putty-like moldable material. Play-Doh is utilized in this model to mimic the shape and consistency of the pancreas parenchyma. When stored in an air-tight container, its moldable consistency is preserved.
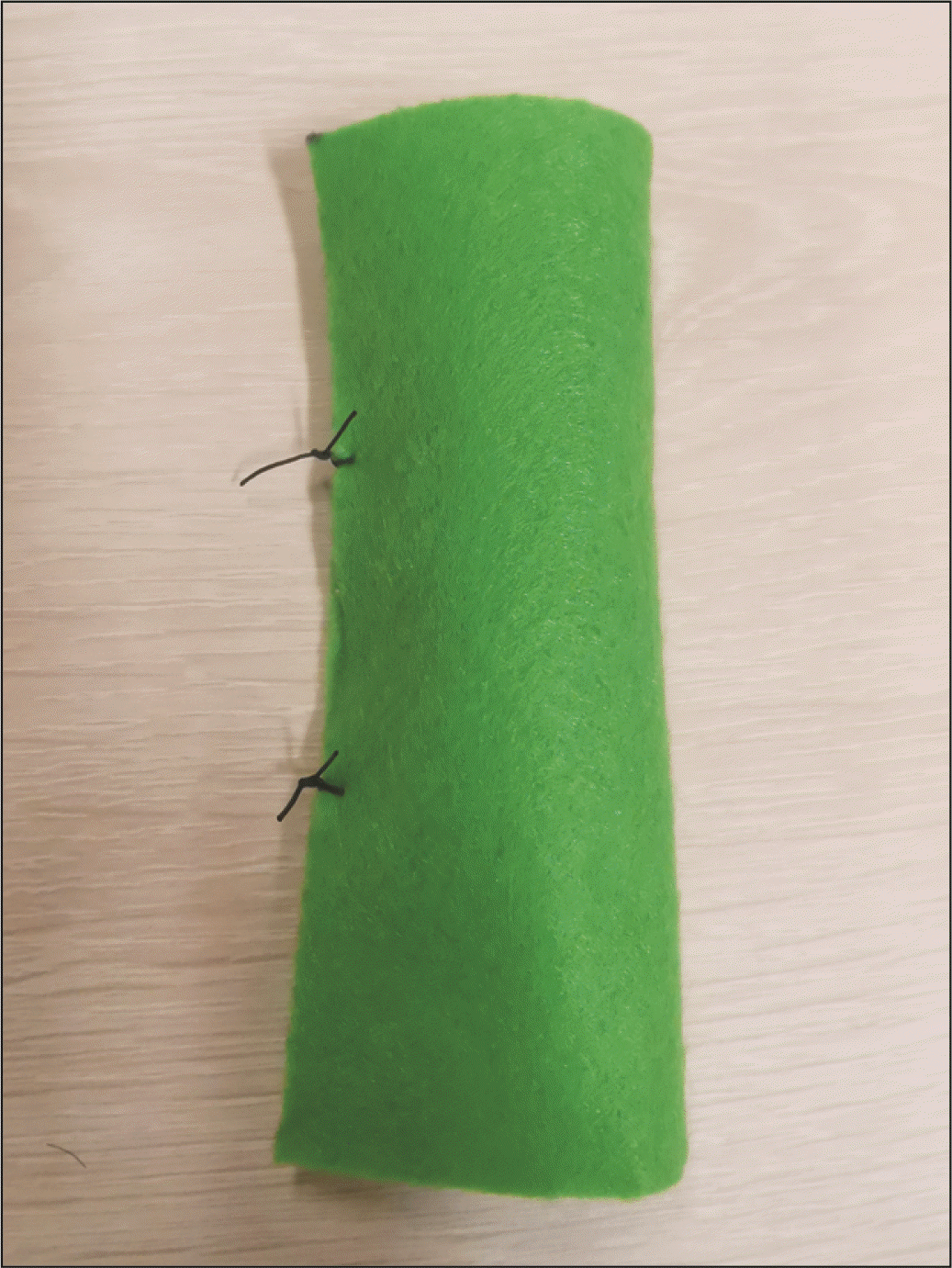
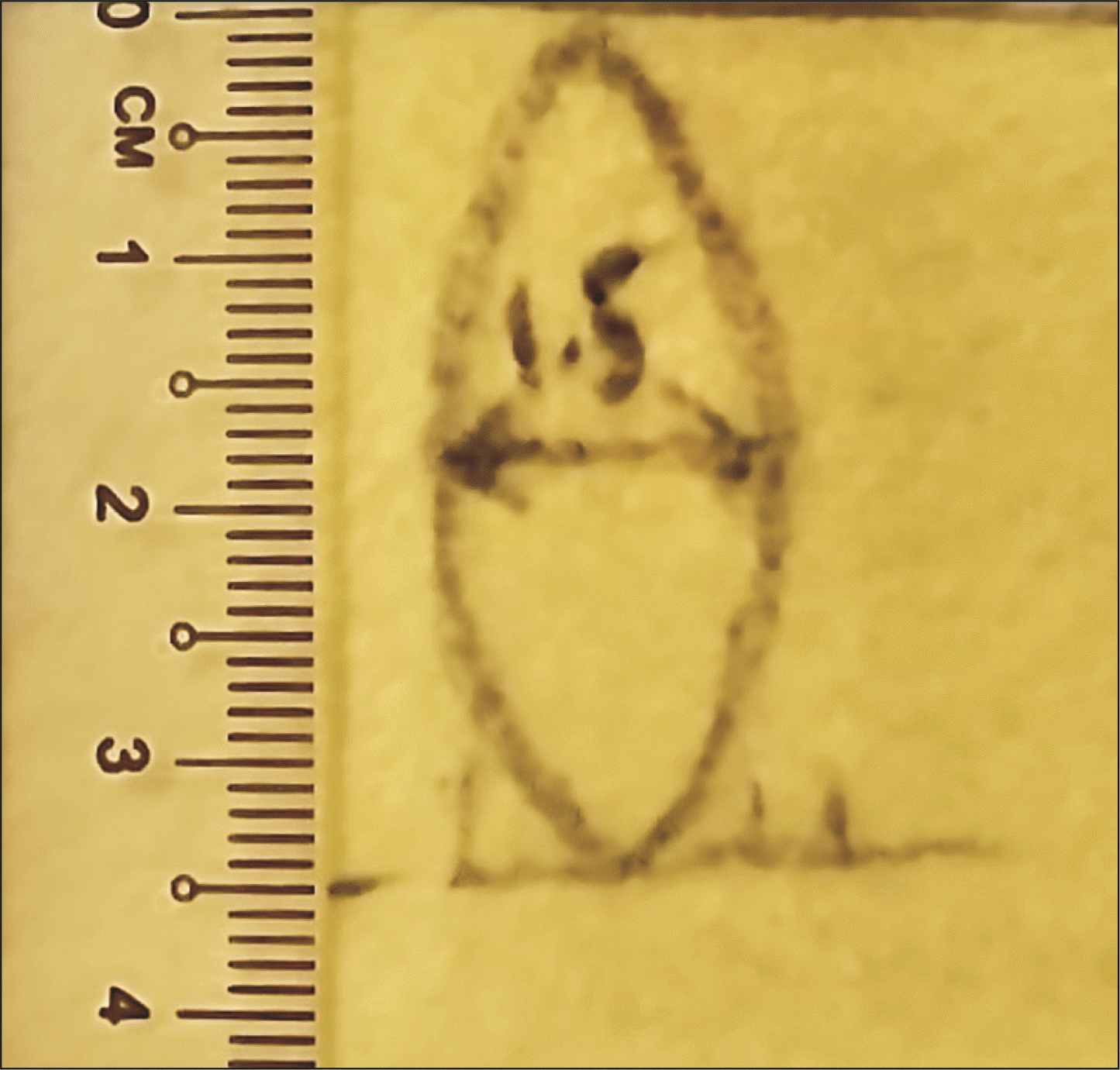
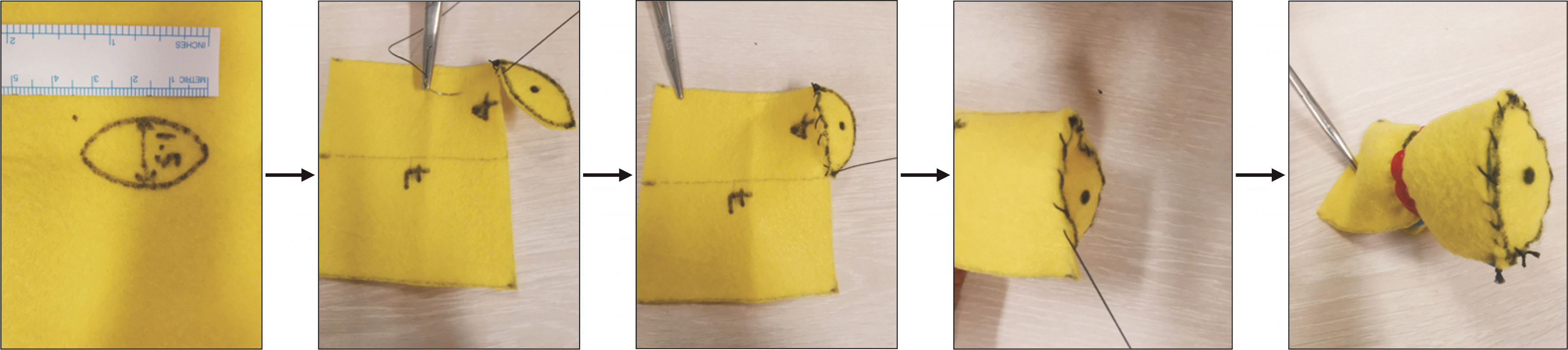
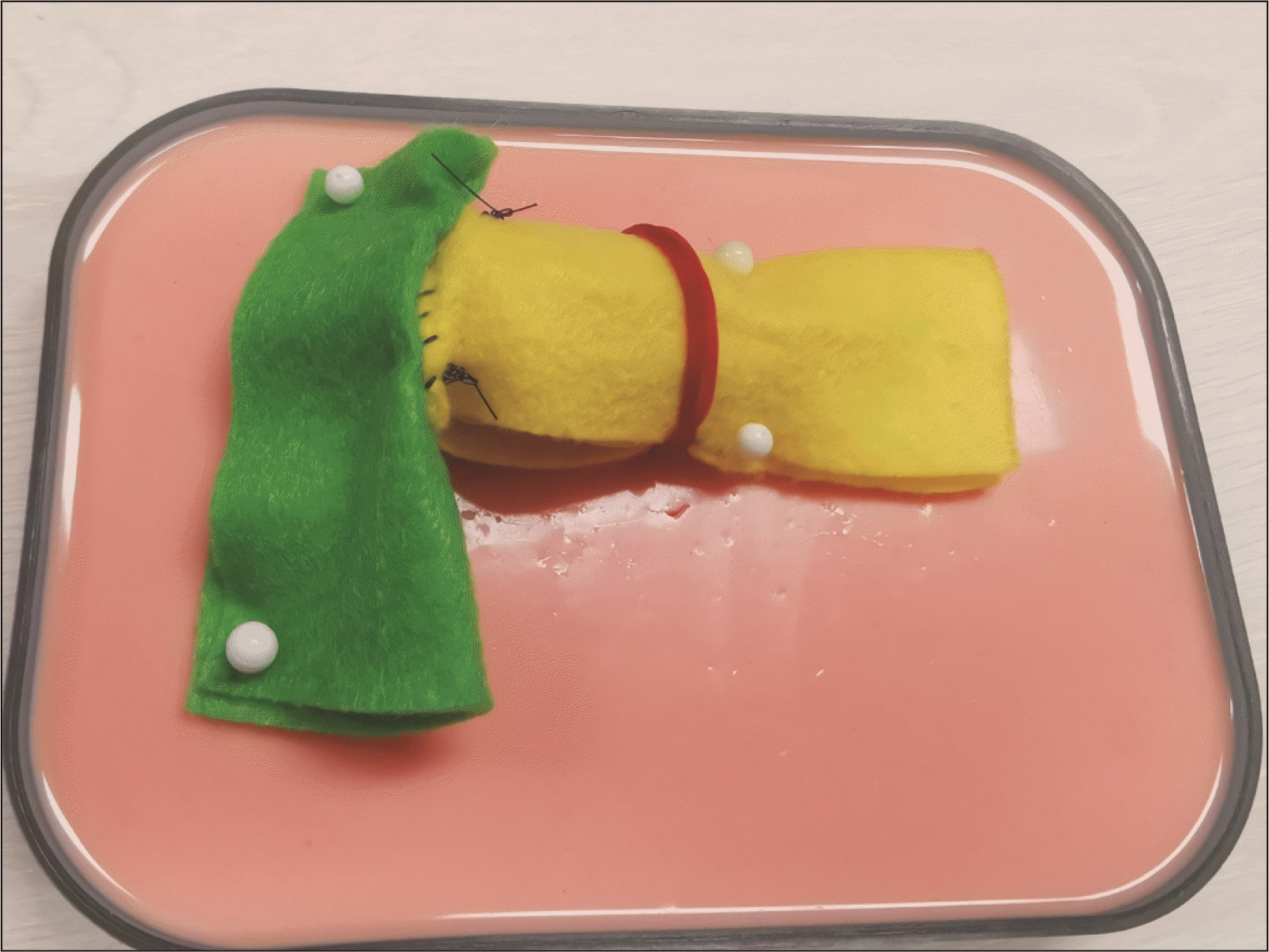
We describe our model construction as follows. Felt fabric (green) is cut to a 8 cm × 10 cm rectangle, then rolled about the long axis, and sutured into a cylinder to mimic the shape of the small bowel (Fig. 1). The pancreas model construction is more meticulous to mimic both the flat cut surface of the pancreas, and the shape of the pancreas body. Firstly, a yellow felt fabric is cut into a 7 cm × 4 cm rectangle, which is then rolled about the short axis and shaped into a cylinder that will act as the conduit for the Play-Doh to be molded into the remnant pancreatic body. Secondly, an additional ellipse of 1.5 cm width and 3.5 cm length is cut from the yellow felt fabric (Fig. 2), and sutured in continuous fashion to one of the openings of the yellow cylinder, which then becomes elliptical in shape (Fig. 3). The pancreatic duct opening is simulated with a mark on the ellipse with an ink pen, and its size can be varied for the purpose of mimicking varying anatomical features.
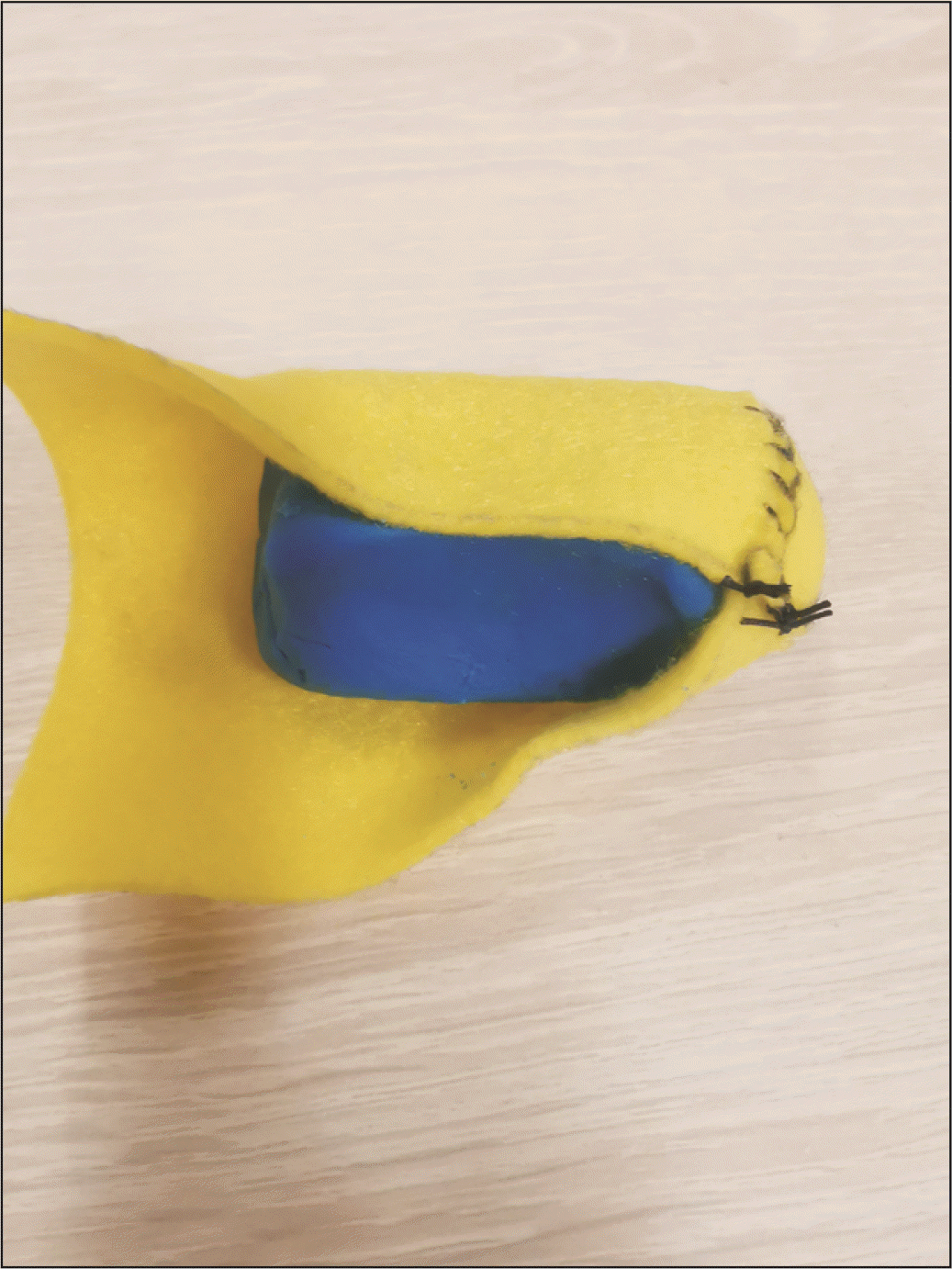
The yellow cylinder is then packed with Play-Doh material, and hand-molded to create a flat surface at the closed elliptical end and the shape of the remnant pancreas body, in preparation for the PJ (Fig. 4). A rubber band is used to maintain the firmness and shape of the model by preventing the Play-Doh from migrating out, and also facilitates the removal of the Play-Doh once the simulation is complete, and the anastomosis taken down. Both the small bowel and pancreas models are then positioned together in the same configuration that mimics intra-corporeal anatomy (Fig. 5). Surgeons can then perform training of MIS PJ with this model in a simulation box. The end-product of this simulation model after MIS PJ is similar to live surgery (Fig. 6). Supplementary Video 1 of the supplementary information (SI) demonstrates that the needle handling and articulation performed during PJ simulation using this model are similar to live robotic surgery.
MIS PD was first reported by laparoscopic approach in 1994 by surgeons Gagner and Pomp, who performed a single, purely laparoscopic procedure [3]. Since then, multiple studies have demonstrated the safety and feasibility of this surgical technique [4-6]. Of late, robotic PD is also increasingly adopted due to the vast advantages of improved articulation, elimination of surgeon tremor, and binocular-enhanced three-dimensional vision [7]. Zeh and Moser published a large series of 132 cases of robotic PD in 2013, demonstrating the safety and feasibility of this technique [7]. Robotic surgery also provides the main surgeon with better ergonomics, hence reducing operative fatigue [8]. Greater magnification and better depth perception allow surgeons to utilize smaller caliber sutures more confidently, but simulation practice in knot-fashioning is required to prevent premature suture snapping as surgeons replace tactile feedback with visual feedback in robotic suturing. As such, many authors describe a difficult and long learning curve for successfully completing MIS PD, especially in functional reconstruction [9].
As with the adoption of any new surgical approach, there exists an initial learning curve, whereby performance outcomes improve with experience. Surgical competency is achieved when the learning curve crosses into acceptable standards, and after an initial peak gradient, often plateaus. A systematic review by Fung et al. [10] reported the median learning curve for laparoscopic PD and robotic PD to be 30 and 36.5 cases, respectively. Speicher et al. [11] reported that to achieve reduction in operative time and blood loss in comparison to an open approach, 50 cases of team-based laparoscopic PD were required for the learning curve. For robotic PD, the reported learning curve is variable. A study published by a Pittsburgh group reported 80 cases of robotic PD were required to overcome the learning curve, whereas a Shanghai group reported reduction in operative time, blood loss, and complications after 40 procedures [12,13]. All the reported studies stated that MIS PD is a highly technical demanding procedure that requires a vast number of cases to overcome the learning curve. Most surgeons reported the performance of PJ anastomosis as one of the technically challenging steps encountered during the reconstructive phase of the operation [2,14,15].
The concern in mounting a learning curve is maintaining clinical outcomes in terms of safety and oncologic measures. High-volume centers have the benefit of caseload and proctorship to facilitate overcoming learning curves [16], whilst other centers have adopted structured training programs, such as LAELAPS−2 and LAELAPS−3 [17]. However, many centers are limited by resources and financial cost, and are unable to replicate such structured training programs; as such, they rely on surgical simulation techniques to improve their practice. Surgical simulation training facilitates an environment free of operating room stressors, without putting patients at risk. Simulators are used for the training of basic, as well as complex, MIS procedures [18]. The limitation of virtual simulation is that often, it is unable to mimic real-life complex operative steps; as such, cadaveric and animal model workshops also exist to facilitate MIS training. Understandably, these workshops come with considerable logistics and resources that add to significant financial cost.
To combat this, bio tissue simulation with bioartificial organs is increasing in popularity. It is a well-recognized form of mastery-based training, and is a part of the training curriculum in the University of Pittsburgh for robotic simulation in PD. Working on that premise, we designed a low-cost do-it-yourself model to mimic the cut pancreas and jejunum specifically to practice the MIS PJ out of the operating theatre.
One of the key benefits to our low-cost model is that the thickness of the pancreas can be varied by adjusting the diameter of the yellow felt cylinder that mimics the pancreas body, and the width of the felt ellipse that mimics the cut surface of the pancreas. The pancreatic duct size can also be adjusted to create different simulation challenges. In addition, once the cylinder and ellipse are sutured together, they can be used multiple times for simulation, as long as the Play-Doh is removed and kept in an air-tight container to maintain its putty-like consistency, as the felt fabric is a fairly versatile textile that can tolerate repeated suturing. This further minimizes the overall time and effort required for subsequent simulation sessions.
Our low-cost model has served as an effective tool in simulating MIS PJ, as can be demonstrated by video S1 of the SI demonstrating side-by-side comparison of bio tissue simulation versus real-life surgery. In our institution, we encourage our surgical trainees with interest in hepato-pancreato-biliary surgery to practice surgical precision and the handling of tissues, as well as articulation using this low-cost model prior to real life surgery. We use this model to allow the trainee to gain familiarity with the steps of PJ, and replicate the angles required for precise suturing. We acknowledge that our model has several limitations. Firstly, unlike bioartificial organs, the model will not be able to mimic the varying consistencies of pancreatic parenchyma tissue. Secondly, this simplified model does not allow the trainee to simulate the insertion of a pancreatic duct stent. Lastly, this model relies on the surgeon to critically assess the quality of the simulated PJ performed—we would advise that the surgeon pay attention to the accuracy of the suture placement for the duct-to-mucosa anastomosis, as well as the snugness of the sutures. As our HPB surgeons utilized this model at different stages of their learning curves and with varying frequencies, we are unable to provide any statistical analysis of their learning curves. Despite these limitations mentioned, we believe that our model is a low-cost tool that has facilitated our journey in performing MIS PD.
Our low-cost and easily constructed model from commercially available products has been useful in simulating MIS PJ creation, as it replicates the challenging intra-corporeal angles and depths of suturing, and is a useful adjunct learning tool for MIS PD. We hope that this model will benefit surgeons who are still on their learning curve in MIS PD.
Supplementary data related to this article can be found at https://youtu.be/cJxStMVDtOM.
REFERENCES
1. Winer J, Can MF, Bartlett DL, Zeh HJ, Zureikat AH. 2012; The current state of robotic-assisted pancreatic surgery. Nat Rev Gastroenterol Hepatol. 9:468–476. DOI: 10.1038/nrgastro.2012.120. PMID: 22733352.
2. Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal M, et al. 2020; Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol. 46:6–14. DOI: 10.1016/j.ejso.2019.08.007. PMID: 31409513.
3. Gagner M, Pomp A. 1994; Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 8:408–410. DOI: 10.1007/BF00642443. PMID: 7915434.
4. Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, et al. 2009; Comparison of laparoscopy-assisted and open pylorus-preserving pancreaticoduodenectomy for periampullary disease. Am J Surg. 198:445–449. DOI: 10.1016/j.amjsurg.2008.12.025. PMID: 19342003.
5. Kim SC, Song KB, Jung YS, Kim YH, Park DH, Lee SS, et al. 2013; Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc. 27:95–103. DOI: 10.1007/s00464-012-2427-9. PMID: 22752284.
6. Pugliese R, Scandroglio I, Sansonna F, Maggioni D, Costanzi A, Citterio D, et al. 2008; Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech. 18:13–18. DOI: 10.1097/SLE.0b013e3181581609. PMID: 18287976.
7. Zeh HJ 3rd, Bartlett DL, Moser AJ. 2011; Robotic-assisted major pancreatic resection. Adv Surg. 45:323–340. DOI: 10.1016/j.yasu.2011.04.001. PMID: 21954697.

8. Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ 3rd. 2013; 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 258:554–559. discussion 559–562. DOI: 10.1097/SLA.0b013e3182a4e87c. PMID: 24002300. PMCID: PMC4619895.
9. Feng Q, Xin Z, Qiu J, Xu M. 2021; Laparoscopic vs. open pancreaticoduodenectomy after learning curve: a systematic review and meta-analysis of single-center studies. Front Surg. 8:715083. DOI: 10.3389/fsurg.2021.715083. PMID: 34568416. PMCID: PMC8461253.

10. Fung G, Sha M, Kunduzi B, Froghi F, Rehman S, Froghi S. 2022; Learning curves in minimally invasive pancreatic surgery: a systematic review. Langenbecks Arch Surg. 407:2217–2232. DOI: 10.1007/s00423-022-02470-3. PMID: 35278112. PMCID: PMC9467952.

11. Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, Blazer DG 3rd, et al. 2014; Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol. 21:4014–4019. DOI: 10.1245/s10434-014-3839-7. PMID: 24923222.

12. Zureikat AH, Postlewait LM, Liu Y, Gillespie TW, Weber SM, Abbott DE, et al. 2016; A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. 264:640–649. DOI: 10.1097/SLA.0000000000001869. PMID: 27433907.

13. Shyr BU, Chen SC, Shyr YM, Wang SE. 2018; Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Medicine (Baltimore). 97:e13000. DOI: 10.1097/MD.0000000000013000. PMID: 30407289. PMCID: PMC6250552.

14. Lee YN, Kim WY. 2018; Comparison of Blumgart versus conventional duct-to-mucosa anastomosis for pancreaticojejunostomy after pancreaticoduodenectomy. Ann Hepatobiliary Pancreat Surg. 22:253–260. DOI: 10.14701/ahbps.2018.22.3.253. PMID: 30215047. PMCID: PMC6125278.

15. Wang W, Zhang Z, Gu C, Liu Q, Liang Z, He W, et al. 2018; The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: a network meta-analysis of randomized control trials. Int J Surg. 57:111–116. DOI: 10.1016/j.ijsu.2018.04.005. PMID: 29777880.

16. Song KB, Kim SC, Lee W, Hwang DW, Lee JH, Kwon J, et al. 2020; Laparoscopic pancreaticoduodenectomy for periampullary tumors: lessons learned from 500 consecutive patients in a single center. Surg Endosc. 34:1343–1352. DOI: 10.1007/s00464-019-06913-9. PMID: 31214805.

17. Zwart MJW, Nota CLM, de Rooij T, van Hilst J, Te Riele WW, van Santvoort HC, et al. Dutch Pancreatic Cancer Group. 2022; Outcomes of a multicenter training program in robotic pancreatoduodenectomy (LAELAPS-3). Ann Surg. 276:e886–e895. DOI: 10.1097/SLA.0000000000004783. PMID: 33534227.

18. Tjønnås MS, Das A, Våpenstad C, Ose SO. 2022; Simulation-based skills training: a qualitative interview study exploring surgical trainees' experience of stress. Adv Simul (Lond). 7:33. DOI: 10.1186/s41077-022-00231-2. PMID: 36273197. PMCID: PMC9588224.





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download