1Department of HPB Surgery, University Hospitals Plymouth NHS Trust, Plymouth, UK
2Department of HPB Surgery, Hospital Clinic de Barcelona, Barcelona, Spain
3Department of HPB Surgery, Hospital Universitari Vall d’Hebron, Barcelona, Spain
4Department of HPB Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
5Department of HPB Surgery, East Lancashire Hospitals NHS Trust, Blackburn, UK
6Department of HPB Surgery, University Hospitals Bristol NHS Foundation Trust, Bristol, UK
7Department of HPB Surgery, The Royal Marsden NHS Foundation Trust, London, UK
8Department of HPB Surgery, University Hospital Coventry & Warwickshire, Coventry, UK
9Department of HPB Surgery, NHS Lothian, Edinburgh, UK
10Department of HPB Surgery, Royal Surrey NHS Foundation Trust, Guildford, UK
11Department of HPB Surgery, Hull University Teaching Hospitals NHS Trust, Hull, UK
12Department of HPB Surgery, Medical University of Innsbruck, Innsbruck, Austria
13Department of HPB Surgery, Ibn Sina Specialized Hospital, Khartoum, Sudan
14Department of HPB Surgery, Shaukat Khanum Memorial Cancer Hospital, Lahore, Pakistan
15Department of HPB Surgery, Leeds Teaching Hospitals NHS Trust, Leeds, UK
16Department of HPB Surgery, Imperial College Healthcare NHS Trust, London, UK
17Department of HPB Surgery, King’s College Hospital NHS Foundation Trust, London, UK
18Department of HPB Surgery, Royal Free London NHS Foundation Trust, London, UK
19Department of HPB Surgery, Monash Medical Centre, Melbourne, Australia
20Department of HPB Surgery, Salvador Zubiran National Institute of Health Sciences and Nutrition, Mexico City, Mexico
21Department of HPB Surgery, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK
22Department of HPB Surgery, Nottingham University Hospitals NHS Trust, Nottingham, UK
23Department of HPB Surgery, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
24Department of HPB Surgery, Policlinico Umberto I University Hospital Sapienza, Rome, Italy
25Department of HPB Surgery, Azienda Ospedaliero Universitaria di Sassari, Sassari, Italy
26Department of HPB Surgery, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
27Department of HPB Surgery, University Hospital Southampton NHS Foundation Trust, Southampton, UK
28Department of HPB Surgery, Swansea Bay University Health Board, Swansea, UK
29Department of HPB Surgery, Hospital Universitario Miguel Servet, Zaragoza, Spain
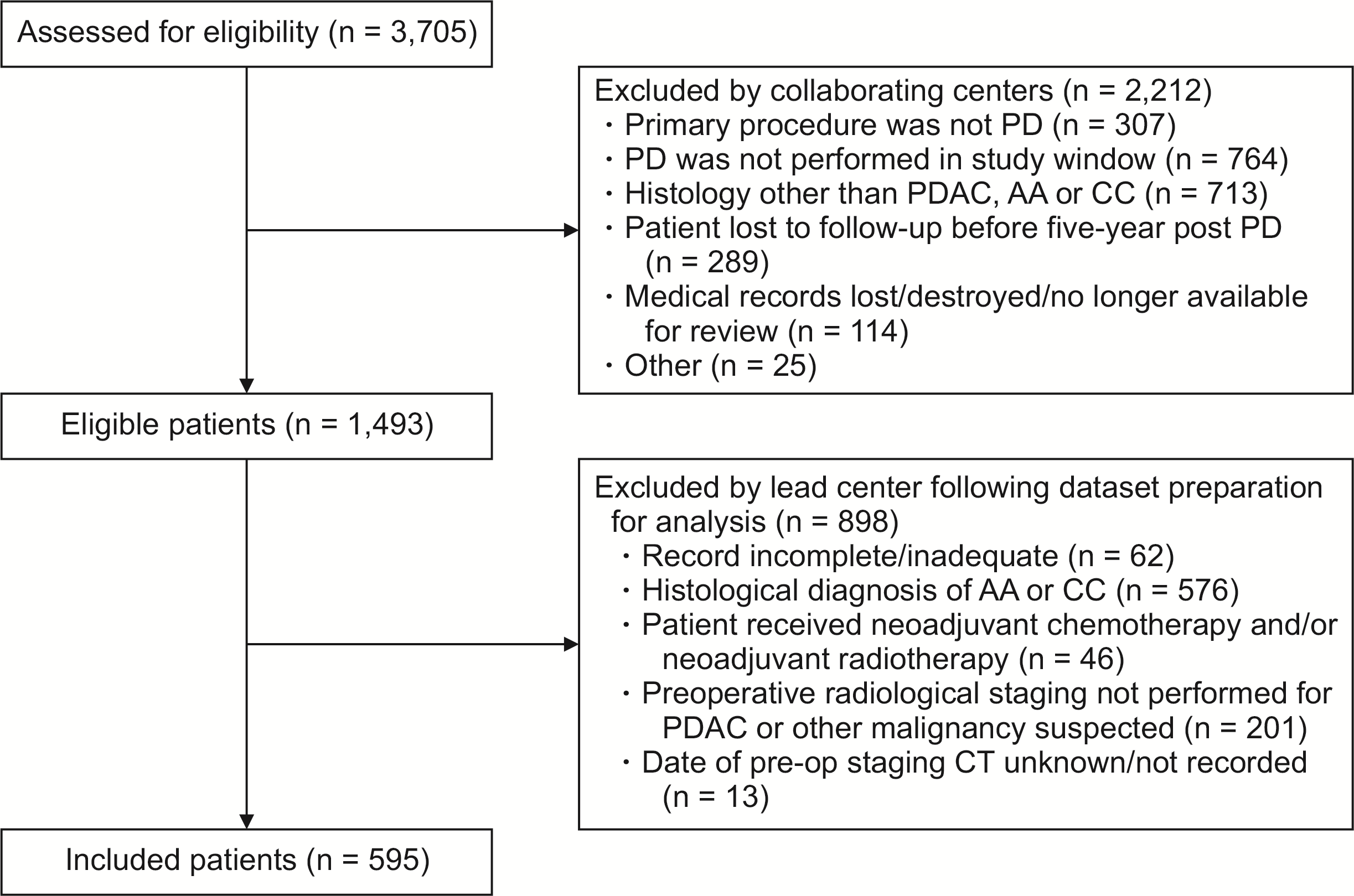
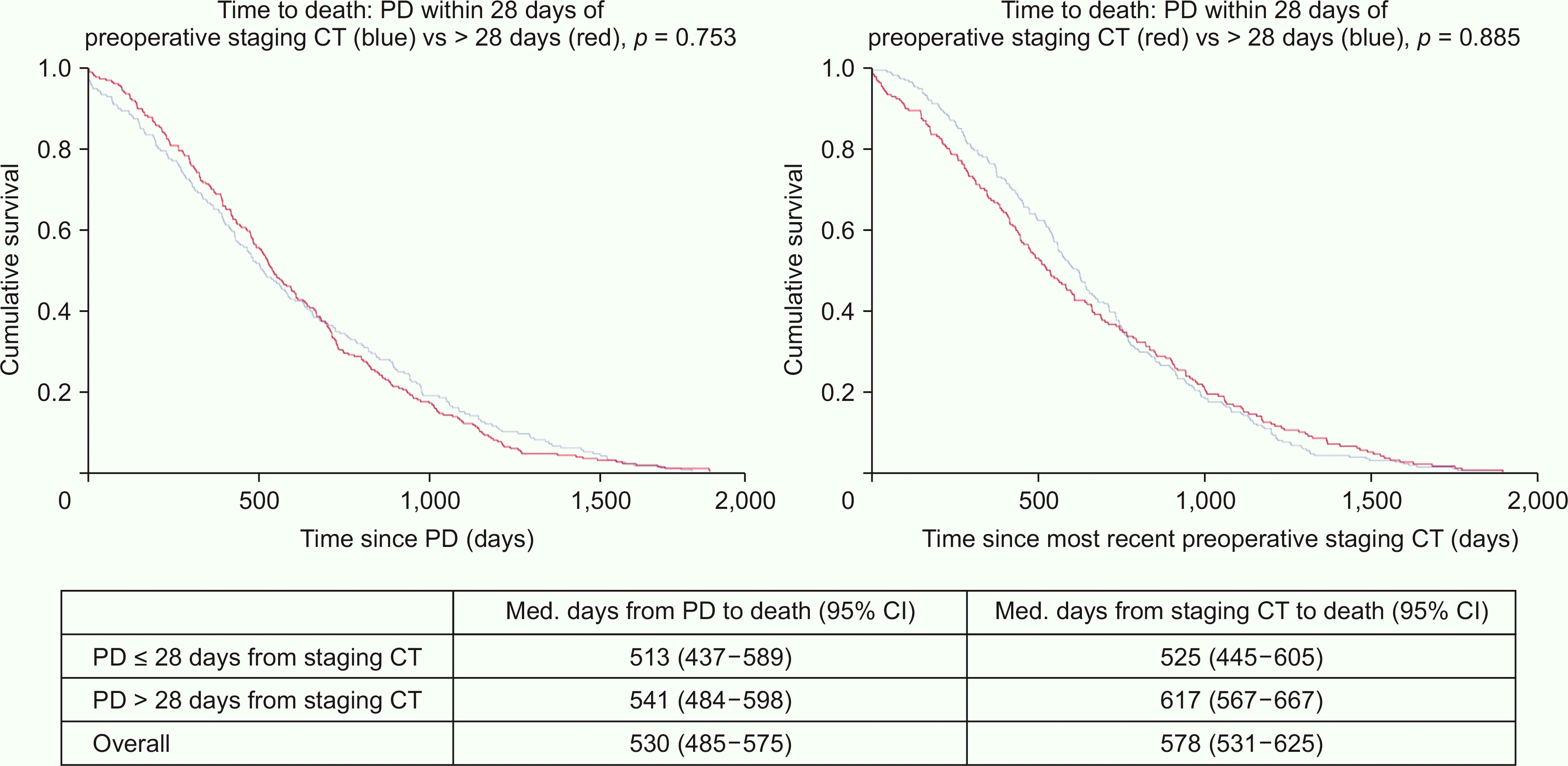




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download