Abstract
Backgrounds/Aims
Although cancer survivors are at higher risk of developing second primary malignancies, cancer surveillance strategies for them have not yet been established. This study aimed to identify first primary cancers that had high risks of developing second primary exocrine pancreatic cancer (EPC).
Methods
Data on individuals diagnosed with primary cancers between 1993 and 2017 were obtained from the Korea Central Cancer Registry. The standardized incidence ratios (SIRs) of second primary EPCs were analyzed according to the primary tumor sites and follow-up periods.
Results
Among the 3,205,840 eligible individuals, 4,836 (0.15%) had second primary EPCs, which accounted for 5.8% of the total EPC patients in Korea. Between 1 and 5 years after the diagnosis of first primary cancers, SIRs of second primary EPCs were increased in patients whose first primary cancers were in the bile duct (males 2.99; females 5.03) in both sexes, and in the small intestine (3.43), gallbladder (3.21), and breast (1.26) in females. Among those who survived 5 or more years after the diagnosis of first primary cancers, SIRs of second primary EPCs were elevated in patients whose first primary cancers were in the bile duct (males 2.61; females 2.33), gallbladder (males 2.29; females 2.22), and kidney (males 1.39; females 1.73) in both sexes, and ovary (1.66) and breast (1.38) in females.
The approximate number of cancer survivors were 18 million in 2022 in the United States, and 2.1 million in 2020 in Korea [1,2]. With the gradual increase in the number of cancer survivors, there is a growing interest in second primary malignancies that contribute to long-term morbidity and mortality [3]. Based on the Surveillance, Epidemiology, and End Results (SEER) database, cancer survivors had a 14% higher risk of developing a subsequent cancer, compared to the general population [4]. Subsequent cancers constituted 18.4% of total incident cancers diagnosed between 2009 and 2013 in the United States [5]. Moreover, patients with second primary malignancies were more likely to die of their second cancers [6].
Approximately 0.2% of digestive tract cancer survivors develop second primary pancreatic cancer [7,8]. Compared to the general population, the standardized incidence ratios (SIRs) of subsequent pancreatic cancers after first primary malignancies at any site ranged (0.65 to 13.14) in the SEER registries [8-10], and (3.08 to 156.78) in the Korean population [7,11]. Due to its dismal prognosis, the development of second primary pancreatic cancer seriously affects the prognosis of cancer survivors. In particular, subsequent pancreatic cancer more than doubled the standardized mortality ratio of first primary cancers of the breast, small intestine, stomach, gallbladder, bile duct, and liver [10]. However, second primary pancreatic cancer has a higher resection rate and better prognosis compared to first primary pancreatic cancer, suggesting that these cancers may have been diagnosed relatively early, due to the enhanced medical surveillance of cancer survivors [7,12].
Exocrine pancreatic cancer (EPC) has a much worse prognosis than neuroendocrine tumors [13]; hence, the risk of subsequent EPC should be the focus of surveillance. This study aimed to investigate the SIRs of second primary EPCs according to first primary tumor sites and follow-up periods using a population-based cancer registry data in Korea, to provide evidence for establishing surveillance strategies in cancer survivors.
The Korea Central Cancer Registry (KCCR) collects cancer incidence data from the entire Korean population in all regions with 98.3% completeness and microscopic verification rate of 92.2% [2,14]. Data obtained from the KCCR between January 1, 1993 and December 31, 2017 were reviewed retrospectively for primary tumor sites and their sequence of diagnosis. The International Association of Cancer Registries defines a primary cancer as “one that originates in a primary site or tissue and is not an extension, nor a recurrence, nor a metastasis” [15]. In the cancer registry, primary tumor sites were classified according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) [16]. According to the SEER code manual that defines simultaneous or synchronous cancers as those diagnosed within 2 months of each other, only subsequent primary cancers that developed at least 2 months after the initial cancers were included in the analysis [4,17]. A cancer survivor is defined as a cancer patient who is alive until the reference date for statistical calculation among patients diagnosed with cancer. Therefore, both patients who are undergoing cancer treatment, and those who have been cured of cancer, are included [2]. This study focused on EPC as a second primary cancer, excluding neuroendocrine tumors that had distinct clinicopathological characteristics, including survival outcomes [13]. EPCs were defined as those with ICD-O-3 codes C250−C259, excluding the following morphology codes for neuroendocrine tumors as described in our previous study: 8150−8157, 8240−8246, 8249, 8002, 8040−8045, and 8013 [18,19]. To analyze the association between second primary EPCs and obesity-related cancers [20], the following subgroups were analyzed as well: breast cancer diagnosed at the age of under versus above 50 years [21]; renal cell carcinoma with ICD-O-3 code C64.9 and ICD-O-3 morphology codes 8260, 8310, 8311, 8312, 8316, 8317, and 8318 [16,22].
The SIR and corresponding 95% confidence intervals (CIs) for subsequent EPCs were assessed to quantify the relative risk compared to that of the general population. SIRs were calculated by dividing the observed number of new EPCs by the number expected, if patients in the cohort experienced the same cancer rates as those in the general reference population of comparable age, sex, and time distribution [4,23]. To reduce bias caused by the immediate excess of second primary cancers due to enhanced medical surveillance after initial cancer diagnosis, analyses were performed according to the follow-up period [24]. The mid-year population reported by Statistics Korea (available at http://kosis.kr/) was used as a general reference population in this study. The person–years at risk for each patient began at 2 months after the date of first primary cancer diagnosis, and ended at the date of death or the end of the study (December 31, 2017), whichever occurred first. Each cancer was analyzed as an independent observation for patients with 2 or more primary cancers. To compute the SIRs and their 95% CIs, we used the “MP−SIR” session of SEER*Stat (version 8.3.8; SEER Program, National Cancer Institute) [25].
The study protocol was reviewed and approved by the Institutional Review Board of the National Cancer Center, which waived the need for informed consent from patients due to the characteristics of the dataset and retrospective nature of the study design (approval No. NCC2021-0251). All research methods were performed in accordance with the Declaration of Helsinki, and the guideline proposed by the Institutional Review Board.
A cohort of 3,205,840 patients diagnosed with first or only primary cancers at any site was identified, 4,836 (0.15%) of whom developed a second primary EPC. Second primary EPCs accounted for 5.8% of a total of 83,640 incident EPC cases during the same period.
Among 3,205,840 patients with primary cancers at any site, the male-to-female ratio was 1.08:1, and 4.5% (n = 144,369) of all the patients had 1 or more subsequent cancers that were diagnosed at least 2 months after the diagnosis of first primary cancer. Mean age at diagnosis was 58.5 years for first primary cancer, and 65.7 years for subsequent cancers. The mean interval between first and subsequent cancers was 5.8 years. Females had their first primary (55.9 [females] vs. 60.9 years [males]) and subsequent cancers (61.6 [females] vs. 68.4 years [males]) diagnosed at earlier ages than males (Supplementary Table 1).
A total of 4,836 patients developed second primary EPC after a mean of 6.6 years after first primary cancer diagnosis at any site. The male-to-female ratio was 1.40:1. Mean age at diagnosis of first primary cancer or subsequent EPC was higher in males (first primary cancer, 63.6 [male] vs. 61.7 [female], p < 0.001; subsequent EPC, 69.9 vs. 69.2 years, p = 0.019). Comparing patients with any subsequent cancers at any site, the mean age at diagnosis of second primary EPC and first primary cancer (69.6 and 62.8 years) was greater than those with any subsequent cancer or their first primary cancer diagnosis (65.7 and 58.5 years), respectively (Table 1, Fig. 1).
During median follow-up for 6.7 years, the relative risk of second primary EPC after first primary cancers of all types was lower than that of the general population (SIR 0.96 [95% CI, 0.94−0.99]). First primary cancer sites that had increased risks of second primary EPCs were bile duct, small intestine, gallbladder, ovary, female breast, and kidney cancers. First primary cancers of the stomach, colon, liver, lymphatic and hematopoietic, prostate, lung, and rectum were less likely to develop second primary EPCs, compared to the general population (Table 2, Supplementary Table 2).
Fig. 2, and Supplementary Table 3 and 4, show the observed cases and SIRs of second primary EPCs according to sex and follow-up periods. In the range (12 to 59) months of follow-up, males had decreased SIRs of second primary EPCs after first primary cancers at all sites (SIR 0.86 [95% CI, 0.82−0.90]), while females did not have significantly different relative risks, compared to the general population. The SIRs of second primary EPCs were increased after bile duct cancer, but decreased after rectal cancer in both sexes. Apart from bile duct cancer, males had decreased SIRs of second primary EPCs after stomach, lung, colon, lymphatic and hematopoietic, prostate, larynx and rectal cancers. Among females, SIRs of second primary EPCs were increased after bile duct, small intestine, gallbladder, and breast cancers. The risks of second primary EPCs were also decreased after stomach, thyroid, and rectal cancers in females.
Among those who survived 5 or more years after the diagnosis of first primary cancers, females had increased SIRs of second primary EPCs after first primary cancers at all sites (SIR 1.13 [95% CI, 1.06−1.19]), while that of males was not statistically different from the general population. The SIRs of second primary EPCs were increased after bile duct, gallbladder, and kidney cancers in both sexes. The SIRs of second primary EPCs after kidney cancer were increased for renal cell carcinoma (SIR 1.58 [95% CI, 1.25−1.97]), but not for other histologies (SIR 0.71 [95% CI, 0.19−1.81]). Males had decreased SIRs of second primary EPCs after rectal cancer. In addition to bile duct, gallbladder, and kidney cancers, females had increased SIRs of second primary EPCs after larynx, ovary, and breast cancers. SIRs of second primary EPCs after breast cancer were increased in all ages (SIR 1.38 [95% CI, 1.21−1.57]), < 50 years (SIR 1.56 [95% CI, 1.21−1.98]), and ≥ 50 years (SIR 1.31 [95% CI, 1.12−1.53]).
First primary bile duct cancer was the only type that had increased SIRs of second primary EPCs throughout the follow-up period in both sexes. In addition, first primary gallbladder and breast cancers had increased SIRs of second primary EPCs throughout the follow-up period in females only. Significantly elevated SIRs of second primary EPC after the diagnosis of breast cancer showed an increasing trend (1.26 to 1.38), while that of gallbladder (3.21 to 2.22) and bile duct cancer (5.03 to 2.33) decreased throughout the follow-up periods.
This study revealed that second primary EPC occurred in 0.15% of all patients with primary cancer at any site, which accounted for 5.8% of the total incident cases of EPC between 1999 and 2017 in Korea. In particular, first primary bile duct and gallbladder cancers were shown to carry increased risks of developing second primary EPC in both sexes (SIR [2.2−5.0]). This finding was consistent with previous reports in the Western population (SIR [2.4−13.1]), and in the Korean population using a different nationwide database (SIR [4.4−156.8]) [7,9,10,24]. In addition to shared anatomy and physiology, there may be genetic traits, lifestyle or environmental factors, and medical conditions that may have had impacts on the development of second primary EPCs as shared determinants among bile duct, gallbladder, and pancreatic cancers [24,26]. However, the SIRs of second primary EPCs after the diagnosis of bile duct and gallbladder cancers decreased throughout the follow-up periods, suggesting a significant impact of extensive medical surveillance during the early follow-up period, or changes in behavioral patterns over time [4].
Decreased risk of second primary EPC after rectal cancer in this study (SIR [0.5−0.8]) reconfirmed the results from the literature (SIR [0.6−0.7]) [8,9,24]. However, the findings on decreased risks of second primary EPC after stomach or colon cancer in this study were contrary to those of other studies in North America, Europe [8,9,24], and Korea [7,11]. Although clarifying etiology was beyond the scope of this study, a few possible interpretations can be proposed. Comparing this study with Western cancer registry studies, differences in incidence and predisposing factors for stomach and colon cancer may have contributed to the discrepancy. Between the Korean studies, direct comparison of results should be made with caution, because data sources and case definitions, including histological, differed. It should be kept in mind that when colon and rectal cancers were analyzed as a group, the effect of increased SIR after colon cancer outweighed the decrease in SIR after rectal cancer [8]. The risk of second primary pancreatic neuroendocrine tumor was significantly increased after stomach and small intestine cancers [27]; however, endocrine tumors were not included in this study.
Those who survived at least 5 years after first primary cancers had a 10%−11% higher risk of developing second primary cancers [10]. In this study, those who survived 5 or more years after first primary cancers at any site had a 6% higher risk of developing second primary EPC. However, cancer surveillance guidelines have yet to be established for cancer survivors, except for post-treatment follow-up protocols for a few primary cancer sites, which recommend cancer screening protocol similar to those for the general population [28,29]. In Korea, the national cancer screening program targets stomach, liver, colon, breast, cervix, and lung cancer. Although participation rates in the program among cancer survivors have continuously increased and reached levels comparable to the general population, there is no evidence-based surveillance guideline for cancer survivors [30]. Pancreatic cancer is not a candidate for population-wide cancer screening. However, the results of this study suggest that survivors of gallbladder, bile duct and kidney cancers in both sexes, and females who have survived larynx, ovary, and breast cancers, should receive more medical surveillance against the possibility of occurrence of second primary EPCs, even after 5 years of follow-up. For example, patients who have completed 5-year follow-up after treatment for their first primary cancer are typically encouraged to undergo the national cancer screening program. Although further investigation is needed, it may be recommended to add abdominal ultrasound or computed tomography to national cancer screening program examinations, if needed, by reminding physicians during national cancer screening consultations that they had a primary cancer with a high risk of subsequent EPC.
Various risk factors, including the treatment of first primary malignancy, lifestyle, environmental, and host factors including genetic susceptibility, as well as interactions between these factors, may contribute to the development of second primary malignancies [31]. Tobacco smoking-related cancers have been repeatedly reported to carry an increased risk of second primary pancreatic cancer, especially in females [4,9,24,32]. This study revealed no significant difference in SIRs of second primary EPC after head and neck, urinary bladder, and cervical cancers; rather, SIRs of second primary EPCs after lung or laryngeal cancer decreased after 12−59 months in males. The relative risks of smoking for lung and kidney cancer have been reported to be lower in Korea, while the risk of lung cancer among never-smokers has been reported to be higher in Asia than in Western countries [33]. Moreover, the risk of developing lung cancer among smokers varied widely according to race, having been shown to be higher in Caucasian and African-Americans, and lower in Asians [34,35]. Although tobacco smoking may not contribute solely to carcinogenesis, this study did not reveal a strong association between smoking-related cancers and second primary EPCs.
Recently, the correlation between metabolic syndrome and the risk of pancreatic cancer has been emphasized [36,37]. Among 12 obesity-related cancers, as designated by the International Agency for Research on Cancer [20], this study revealed increased SIRs of second primary EPCs after gallbladder, bile duct, kidney, breast, and ovarian cancers. For kidney cancer, the significance of renal cell carcinoma, which was classified as an obesity-related cancer, was shown in this study, while kidney cancer with other histology had nonsignificant differences in SIRs, compared to the general population [20]. In contrast to the findings of previous studies that reported an elevated risk of second primary EPCs among younger breast cancer patients [9,24], our cohort had elevated risk in both those diagnosed under and above 50 years of age. This study revealed a decreased risk of second primary EPC after colon cancer, especially between 12 and 59 months in males, in contrast to some European and North American studies that reported an increased risk of EPC after colon cancer [8,9,24].
This study has strengths, as well as limitations. In Korea, the KCCR and the National Health Insurance Service database are most commonly used for population-based analysis. However, cancer diagnosis based on the operational definition of the latter matched less than 90% of that on the KCCR, with lower accuracy in diagnostic information of multiple primary cancer [13,38]. As described in our methods section, the strict registration protocol of the cancer registry improved the quality of our multiple primary cancer analysis, excluding recurrence or metastasis. Nevertheless, the KCCR has reported nationwide cancer statistics since 1999; hence, information on primary cancers that occurred before 1999 might have not been included in our dataset. Morphological codes were used to select for EPCs from all cancers originating in the pancreas; however, radiographic information on which to base clinical diagnosis could not be identified in cases without microscopic verification [39]. Due to limitations on variables in the cancer registry, lifestyle, environmental, and host factors associated with second primary EPCs could not be evaluated. In addition, intensive cancer surveillance after the diagnosis of first primary cancers may have increased the detection of second primary cancers in early follow-up periods.
This study investigated the relative risks of second primary EPCs after first primary cancers at all sites, to provide evidence for surveillance guidelines for Asian cancer survivors. Cancers of the bile duct and gallbladder, which share anatomical structures and physiologies, and obesity-related cancers including breast, ovarian, and kidney renal cell carcinoma, were shown to carry an increased risk of second primary EPCs. In contrast, unlike the increased risk of second primary EPCs after lung, larynx, and colon cancers in Western populations, the link observed in this study was minimal or decreased. Close medical attention should be paid to cancer survivors of bile duct, gallbladder, breast, ovary, and kidney cancers, due to the higher risks of their developing second primary EPCs, even after 5 years of follow-up.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.23-053.
REFERENCES
1. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. 2022; Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436. DOI: 10.3322/caac.21731. PMID: 35736631.
2. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2019. Goyang: Ministry of Health and Welfare;2021. Dec. Report No.: 11-1352000-000145-10.
3. Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. 2012; Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 30:3734–3745. DOI: 10.1200/JCO.2012.41.8681. PMID: 23008293.
4. Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, et al. 2006. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. National Cancer Institute;Bethesda, MD: NIH Publ. No. 05-5302. DOI: 10.1200/jco.2012.41.8681.
5. Murphy CC, Gerber DE, Pruitt SL. 2018; Prevalence of prior cancer among persons newly diagnosed with cancer: an initial report from the surveillance, epidemiology, and end results program. JAMA Oncol. 4:832–836. DOI: 10.1001/jamaoncol.2017.3605. PMID: 29167866. PMCID: PMC6370034.
6. Donin N, Filson C, Drakaki A, Tan HJ, Castillo A, Kwan L, et al. 2016; Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 122:3075–3086. DOI: 10.1002/cncr.30164. PMID: 27377470. PMCID: PMC6192520.

7. Ahn HS, Kang TU, Swan H, Kang MJ, Kim N, Kim HJ, et al. 2019; Incidence and mortality rates of second pancreatic cancer among survivors of digestive cancers: a nationwide population-based study. Pancreas. 48:412–419. DOI: 10.1097/MPA.0000000000001254. PMID: 30768577.

8. Rahimi E, Batra S, Thosani N, Singh H, Guha S. 2016; Increased incidence of second primary pancreatic cancer in patients with prior colorectal cancer: a population-based US study. Dig Dis Sci. 61:1652–1660. DOI: 10.1007/s10620-016-4170-x. PMID: 27107866.

9. Amin S, McBride RB, Kline JK, Mitchel EB, Lucas AL, Neugut AI, et al. 2012; Incidence of subsequent pancreatic adenocarcinoma in patients with a history of nonpancreatic primary cancers. Cancer. 118:1244–1251. DOI: 10.1002/cncr.26414. PMID: 21887676. PMCID: PMC3677019.

10. Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A. 2020; Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA. 324:2521–2535. DOI: 10.1001/jama.2020.23130. PMID: 33351041. PMCID: PMC7756242.

11. Chung JW, Chung MJ, Bang S, Park SW, Song SY, Chung JB, et al. 2017; Assessment of the risk of colorectal cancer survivors developing a second primary pancreatic cancer. Gut Liver. 11:728–732. DOI: 10.5009/gnl16526. PMID: 28750486. PMCID: PMC5593336.

12. Jo JH, Cho IR, Jung JH, Lee HS, Chung MJ, Bang S, et al. 2017; Clinical characteristics of second primary pancreatic cancer. PLoS One. 12:e0179784. DOI: 10.1371/journal.pone.0179784. PMID: 28650984. PMCID: PMC5484482.

13. Nikšić M, Matz M, Valkov M, Marcos-Gragera R, Stiller C, Rosso S, et al. 2022; World-wide trends in net survival from pancreatic cancer by morphological sub-type: an analysis of 1,258,329 adults diagnosed in 58 countries during 2000-2014 (CONCORD-3). Cancer Epidemiol. 80:102196. DOI: 10.1016/j.canep.2022.102196. PMID: 35841761.

14. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. 2022; Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 54:330–344. DOI: 10.4143/crt.2022.128. PMID: 35313102. PMCID: PMC9016309.

15. Working Group Report. 2005; International rules for multiple primary cancers (ICD-0 third edition). Eur J Cancer Prev. 14:307–308. DOI: 10.1097/00008469-200508000-00002. PMID: 16030420.
16. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. 2000. International classification of diseases for oncology, 3rd ed [Internet]. Available from: https://apps.who.int/iris/handle/10665/42344. cited 2022 Oct 27.
17. Fritz A. SEER program code manual. third edition. National Cancer Institute;1998. DOI: 10.1097/00008469-200508000-00002.
18. Man D, Wu J, Shen Z, Zhu X. 2018; Prognosis of patients with neuroendocrine tumor: a SEER database analysis. Cancer Manag Res. 10:5629–5638. DOI: 10.2147/CMAR.S174907. PMID: 30519109. PMCID: PMC6239108.

19. Kang MJ, Lim J, Han SS, Park HM, Park SJ, Won YJ, et al. 2022; First course of treatment and prognosis of exocrine pancreatic cancer in Korea from 2006 to 2017. Cancer Res Treat. 54:208–217. DOI: 10.4143/crt.2021.421. PMID: 34030432. PMCID: PMC8756130.

20. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. International Agency for Research on Cancer Handbook Working Group. 2016; Body fatness and cancer-viewpoint of the IARC Working Group. N Engl J Med. 375:794–798. DOI: 10.1056/NEJMsr1606602. PMID: 27557308. PMCID: PMC6754861.

21. Park CY, Lim JY, Park HY. 2018; Age at natural menopause in Koreans: secular trends and influences thereon. Menopause. 25:423–429. DOI: 10.1097/GME.0000000000001019. PMID: 29112598.

22. National Cancer Institute. 2020. Surveillance, Epidemiology, and End Results Program. Kidney;Solid tumor rules: DOI: 10.1097/gme.0000000000001019.
23. Begg CB. Neugut AI, Meadows AT, Robinson E, editors. Methodological and statistical considerations in the study of multiple primary cancers. Multiple Primary Cancers. Lippincott Williams & Wilkins;p. 13–26.
24. Shen M, Boffetta P, Olsen JH, Andersen A, Hemminki K, Pukkala E, et al. 2006; A pooled analysis of second primary pancreatic cancer. Am J Epidemiol. 163:502–511. DOI: 10.1093/aje/kwj073. PMID: 16421239.

25. Min SK, Choi SW, Lim J, Park JY, Jung KW, Won YJ. 2019; Second primary cancers in patients with oral cavity cancer included in the Korea Central Cancer Registry. Oral Oncol. 95:16–28. DOI: 10.1016/j.oraloncology.2019.05.025. PMID: 31345385.

26. Lin Y, Tamakoshi A, Kawamura T, Inaba Y, Kikuchi S, Motohashi Y, et al. 2002; Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. Int J Cancer. 99:742–746. DOI: 10.1002/ijc.10402. PMID: 12115510.

27. Kamath GR, Kim MK, Taioli E. 2019; Risk of primary neuroendocrine pancreatic tumor after a first primary cancer: a US population-based study. Pancreas. 48:161–168. DOI: 10.1097/MPA.0000000000001232. PMID: 30589832. PMCID: PMC6331272.

28. National Comprehensive Cancer Network. 2022. NCCN clinical practice guidelines in oncology: Survivorship, version 1. Available from: https://www.nccn.org/guidelines. cited 2022 Dec 2.
29. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. 2016; American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 34:611–635. DOI: 10.1200/JCO.2015.64.3809. PMID: 26644543.

30. Yun EH, Hong S, Her EY, Park B, Suh M, Choi KS, et al. 2020; Trends in Participation Rates of the National Cancer Screening Program among Cancer Survivors in Korea. Cancers (Basel). 13:81. DOI: 10.3390/cancers13010081. PMID: 33396692. PMCID: PMC7796127.

31. Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, et al. 2006; Cancer survivorship-genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 98:15–25. DOI: 10.1093/jnci/djj001. PMID: 16391368.

32. Neugut AI, Ahsan H, Robinson E. 1995; Pancreas cancer as a second primary malignancy. A population-based study. Cancer. 76:589–592. DOI: 10.1002/1097-0142(19950815)76:4<589::AID-CNCR2820760408>3.0.CO;2-9. PMID: 8625151.

33. Park S, Jee SH, Shin HR, Park EH, Shin A, Jung KW, et al. 2014; Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer. 14:406. DOI: 10.1186/1471-2407-14-406. PMID: 24902960. PMCID: PMC4090397.

34. Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, et al. 2008; Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 122:155–164. DOI: 10.1002/ijc.23033. PMID: 17893872.

35. Park S, Bae J, Nam BH, Yoo KY. 2008; Aetiology of cancer in Asia. Asian Pac J Cancer Prev. 9:371–380. PMID: 18990005.
36. Zhong L, Liu J, Liu S, Tan G. 2023; Correlation between pancreatic cancer and metabolic syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 14:1116582. DOI: 10.3389/fendo.2023.1116582. PMID: 37113491. PMCID: PMC10126301.

37. Xia B, He Q, Pan Y, Gao F, Liu A, Tang Y, et al. 2020; Metabolic syndrome and risk of pancreatic cancer: a population-based prospective cohort study. Int J Cancer. 147:3384–3393. DOI: 10.1002/ijc.33172. PMID: 32580250.

38. Kim BW, Oh CM, Choi HY, Park JW, Cho H, Ki M. Incidence and overall survival of biliary tract cancers in South Korea from 2006 to 2015: using the National Health Information Database. Gut Liver. 2019; 13:104–113. DOI: 10.5009/gnl18105. PMID: 29938462. PMCID: PMC6347005.

39. Hwang YJ, Park SM, Ahn S, Lee JC, Park YS, Kim N. 2019; Accuracy of an administrative database for pancreatic cancer by international classification of disease 10th codes: a retrospective large-cohort study. World J Gastroenterol. 25:5619–5629. DOI: 10.3748/wjg.v25.i37.5619. PMID: 31602162. PMCID: PMC6785515.

Fig. 1
Distribution of age group at diagnosis of first primary and subsequent cancer. EPC, exocrine pancreatic cancer.
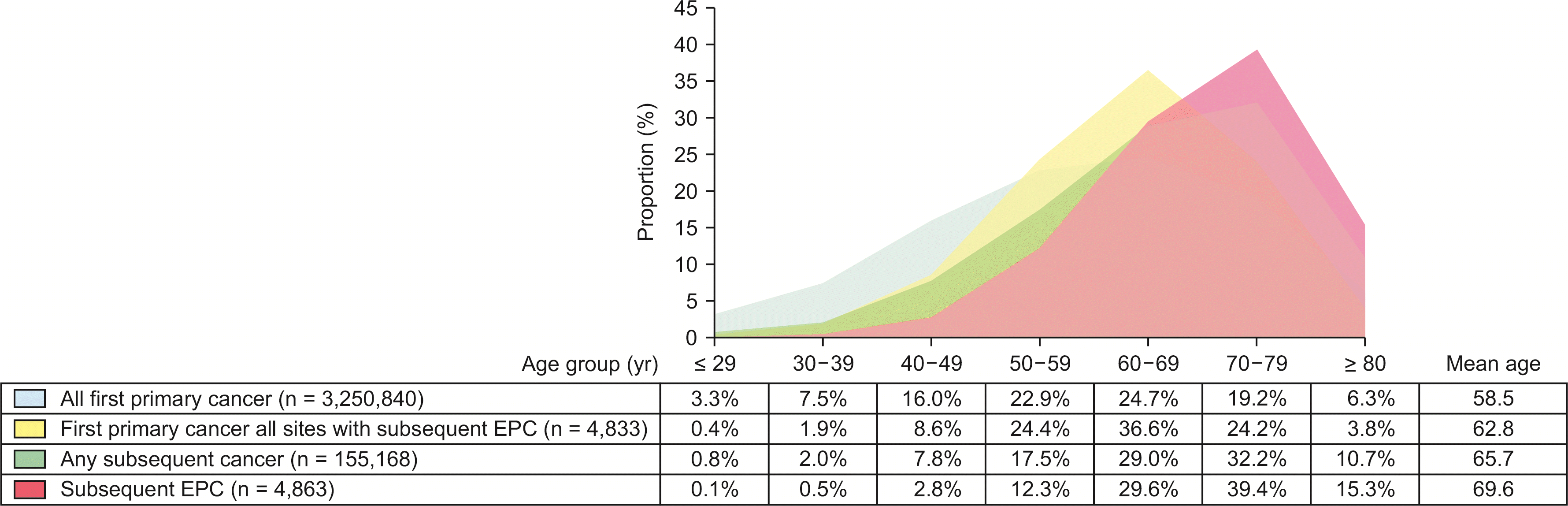
Fig. 2
Observed cases and statistically significant SIRs of secondary exocrine pancreatic cancer by sex and follow-up period. (A) Summary. (B) Male. (C) Female. SIR, standardized incidence ratio.
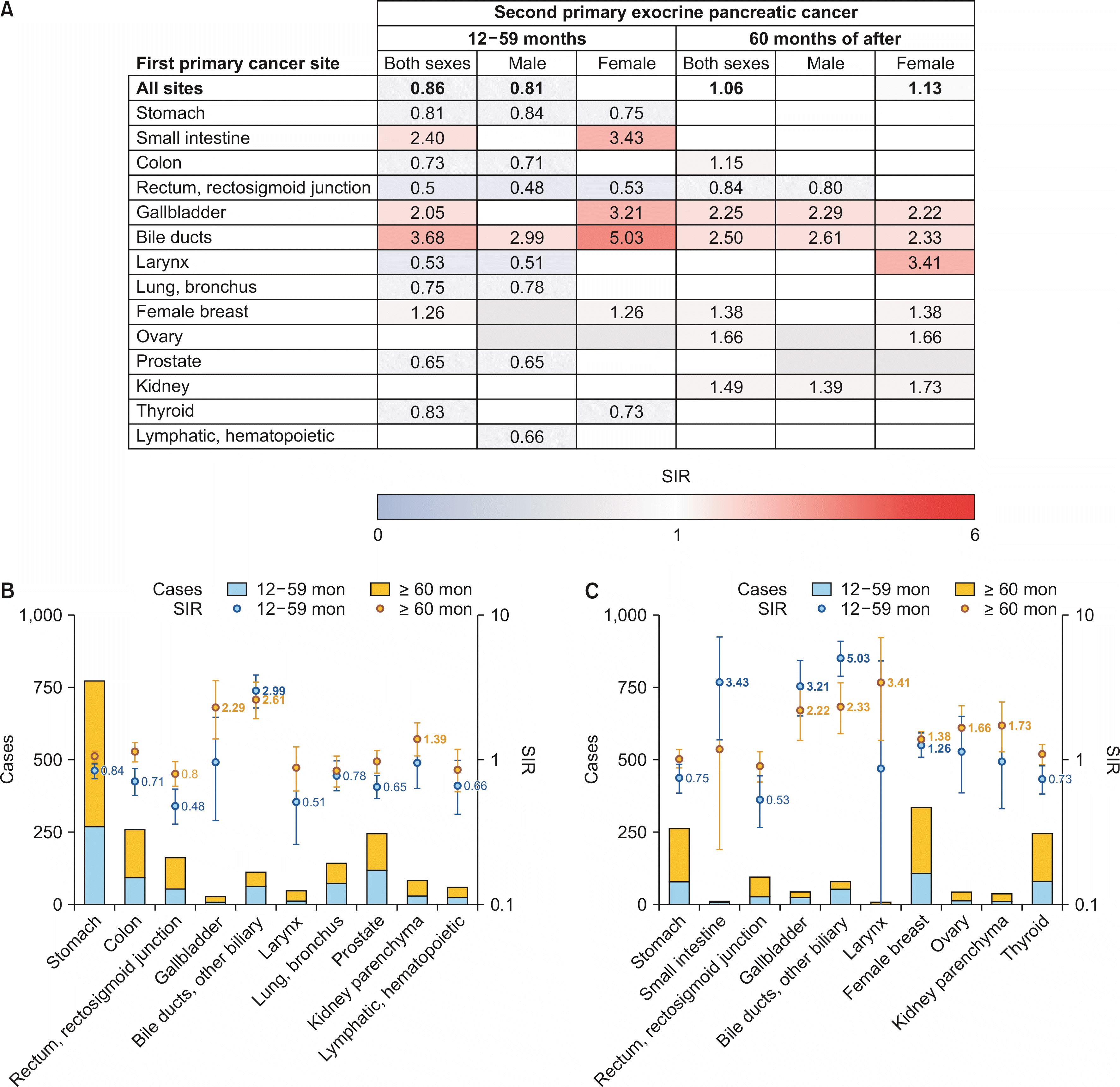
Table 1
Characteristics of patients with primary cancer of all sites with second primary EPC (1993−2017)
Table 2
SIRs of second primary exocrine pancreatic cancer in both sexes after first primary cancer at all sites




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download