This article has been corrected. See "Corrigendum: Clinicopathological characteristics of extrahepatic biliary neuroendocrine neoplasms in the gallbladder, extrahepatic biliary tract, and ampulla of Vater: A single-center cross-sectional study" in Volume 28 on page 114.
Abstract
Backgrounds/Aims
In 2019, the grading and staging system for neuroendocrine neoplasms (NENs) was significantly changed. In this study, we report the clinicopathological characteristics and surgical outcomes of patients with extrahepatic biliary NENs who underwent curative resection with or without adjuvant treatment.
Methods
We retrospectively reviewed a database of 16 patients who developed NENs, neuroendocrine carcinoma (NEC), and mixed endocrine non-endocrine neoplasms (MiNENs) after curative resection. Among them, eight patients had ampulla of Vater (AoV) tumors, and eight patients had non-AoV tumors.
Results
G1 and G2 were more frequently observed in the AoV group than in the non-AoV group (12.5% and 62.5%, respectively). In contrast, NEC and MiNEN were more common in the non-AoV group (50.0%). High Ki-67 index (> 20%) and perineural invasion (PNI) were more frequently observed in the non-AoV group. Advanced age (> 65 years), mitotic count > 20 per 2 mm2, and Ki-67 index > 20% were strongly correlated with patient survival (p = 0.018, 0.009, and 0.044, respectively). Advanced age (> 65 years) and mitotic count > 20 per 2 mm2 were significantly correlated with disease recurrence (p = 0.033 and 0.010, respectively).
Conclusions
AoV and non-AoV tumors had significant differences in the histologic grade, Ki67, and PNI. Patients with non-AoV tumors had an increased risk for survival and recurrence than those in the AoV group. For extrahepatic biliary NENs, early detection of tumors, adequate surgery, and aggressive adjuvant treatment for high-risk patients are important to achieve long-term survival and prevent disease recurrence.
The incidence rate of neuroendocrine neoplasms (NENs) is increasing at an annual rate of 6% [1]. NENs mostly occur in the gastrointestinal tract (66%) or the bronchopulmonary system (31%), but they can also occur in the ovaries, testes, hepatobiliary system, and pancreas [1]. Extrahepatic bile ducts are rare primary sites of NENs, accounting for only 0.2%–2% of all such malignancies [2,3]. Table 1 shows the classification and grading of NENs based on the 2019 World Health Organization (WHO) classification. NENs are classified as Grade 1 (G1) (mitotic count < 2 per 2 mm2 and/or < 3% Ki-67 index), Grade 2 (G2) (mitotic count 2–20 per 2 mm2 and/or 3%–20% Ki-67 index), and Grade 3 (G3) (mitotic count > 20 per 2 mm2 and/or > 20% Ki-67 index). Neuroendocrine carcinoma (NEC), including small-cell and large-cell types, is defined as high grade and poorly differentiated NENs [4]. A mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) is defined when each component is morphologically and immunohistochemically recognizable and constitutes ≥ 30% of the tumor burden. NEC is a separate disease entity from well-differentiated neuroendocrine tumors because it has different clinicopathological features, prognoses, and molecular alterations [5]. All NENs have malignant potential. Extrahepatic biliary NENs, except those in the ampulla of Vater (AoV), are difficult to diagnose preoperatively and clearly distinguish from cholangiocarcinomas. There is no standard guideline for treatment of extrahepatic biliary NENs because their natural characteristics and prognostic factors remain unclear; however, to date, aggressive multimodal treatments are known to be the only treatment to increase the survival rate [6,7]. In this study, we report the clinicopathological characteristics and surgical outcomes of patients with extrahepatic biliary NENs (AoV, gallbladder [GB], and extrahepatic biliary tract) who underwent curative resection with or without adjuvant treatment.
A total of 16 patients diagnosed with NENs or MiNENs underwent curative resection with or without adjuvant treatment between 2010 and 2020 at Pusan National University Hospital. One patient was classified as having G1, five had G2, and none of the patients had G3. Five patients each were diagnosed with NEC (small-cell type) and MiNEN, respectively. Large-cell type NEC was not included in our study. Patients’ data were retrospectively reviewed after approval was obtained from the relevant institutional review board. The requirement for obtaining informed consent from patients was waived due to the retrospective nature of the study. This retrospective study was performed in accordance with the relevant guidelines and regulations, and it was approved by the Pusan National University Institutional Review Board at Clinical Trial Center (Institutional Review Board number: 2109-004-107).
The type of surgery decided by the surgeon was dependent on the tumor location. Pancreatoduodenectomy was performed to resect tumors located in the AoV and distal common bile duct. Extended cholecystectomy was performed to resect tumors located in the GB, and radical bile duct resection was performed to resect mid-distal common bile duct tumors. Lymphadenectomy from the left celiac trunk to the hepatoduodenal ligament, including the retropancreatic region, was routinely performed. If metastasis or direct invasion was suspected, additional tissues were resected.
Histopathological diagnosis was determined based on the following diagnostic criteria: (1) positive immunohistochemical staining of multiple proteins, including chromogranin A and synaptophysin, or the cluster of differentiation, which indicates the presence of neural cell adhesion molecules, and (2) histopathologic presence of high-grade and small-cell cytologic features, very high cellularity with hyperchromatic nuclei, absence of very small nucleoli with scant cytoplasm, high nuclear-cytoplasmic ratio, and round- or fusiform-shaped cells (based on the classification of squamous cell carcinomas as NENs by the WHO) [6,8-11].
Adjuvant chemotherapy was administered to treat lymph node (LN) metastasis, lymphovascular invasion (LVI), or perineural invasion (PNI) among patients with G1 and G2 tumors, or in patients with NECs or MiNENs. The chemotherapy regimen included etoposide and cisplatin. In patients with dominant adenocarcinoma in combined adenoneuroendocrine carcinoma, gemcitabine and cisplatin or 5-fluorouracil and leucovorin were administered. Combined chemoradiation therapy was also administered to patients in the adenocarcinoma-dominant group.
Categorical variables of the AoV and non-AoV groups were compared, and differences were analyzed using log-rank tests. A two-tailed Fisher’s exact test was used to compare the categorical variables. Statistical significance was set at p < 0.05. Age, tumor size, and LN ratio were compared using Student’s t-test and Mann-Whitney U test. Overall survival (OS) and disease-free survival (DFS) were estimated according to the Kaplan-Meier method, and survival differences were evaluated using the log-rank test. This work was supported by the Department of Biostatistics, Biomedical Research Institute, Pusan National University Hospital. Statistical analyses were performed using the SPSS software version 20.0 (IBM Corp.).
The mean age of patients was 57.88 ± 13.27 years (range, 36 to 74 years). The non-AoV group had more patients above 65 years of age than the AoV group (62.5% vs. 12.5%). Overall, 56.2% of patients were females and 43.8% of patients were males, respectively. Six patients presented with symptoms, whereas the remaining patients (62.5%) were incidentally diagnosed during health screening or evaluation for other diseases. There was a significant difference in histologic grades between the two groups. In the AoV group, G1 and G2 were more frequently observed than in the non-AoV group (12.5% and 62.5% vs. 0% and 0%, respectively, p = 0.022). In contrast, NEC and MiNEN were more common in the non-AoV group (50% vs. 12.5%, respectively, p = 0.022). High Ki-67 index (> 20%) and PNI were more frequent in the non-AoV group (25% vs. 100%, p = 0.002; and 87.5% vs. 0%, p < 0.001, respectively). There were no significant differences in the mitosis, T status, LN metastasis, and LVI (Table 2).
The median follow-up period after surgery was 72 months. The 1-, 3-, and 5-year postoperative OS rates were 87.5%, 81.3%, and 72.2%, respectively (Fig. 1A). The 1-, 3-, and 5-year DFS rates were 68.8%, 62.5%, and 62.5%, respectively (Fig. 1B).
In the survival comparison of the AoV and non-AoV groups, there was no significant difference between the two groups (the 1-, 3-, and 5-year postoperative OS rates: 87.5%, 87.5%, and 72.9%; and 87.5%, 75.0%, and 75.0%, p = 0.782, respectively) (Fig. 2A). In the DFS, there was no significant difference between the two groups (the 1-, 3-, and 5-year postoperative DFS rates: 75.0%, 62.5%, and 62.5%; and 62.5%, 62.5%, and 62.5%, p = 0.869, respectively) (Fig. 2B).
In the analysis of OS, advanced age > 65 years, mitotic count > 20 per 2 mm2, and Ki-67 index > 20% were strongly correlated with patient survival (p = 0.018, 0.009, and 0.044, respectively). However, the histologic grade of NEC and MiNENs, and T status were associated with prognosis, but there were no statistical significances (p = 0.065 and 0.069, respectively). LN metastasis, LVI, and/or PNI were not significantly associated with prognosis. In the analysis of DFS, advanced age > 65 years and mitotic count > 20 per 2 mm2 were significantly correlated with disease recurrence (p = 0.033 and 0.010, respectively). According to multivariate analysis, mitotic count > 20 per 2 mm2 was a significant predictor for poor OS and DFS (hazard ratio = 17.48, p = 0.033; and hazard ratio = 17.66, p = 0.033, respectively) (Table 3).
Table 4 shows, in brief, the clinicopathologic characteristics and outcomes of each patient with NEN. One patient (case #6) had a G2 lesion with single hepatic metastasis. He survived without any recurrence for 32 months postoperatively without adjuvant treatment. Recurrence was observed in six patients. The first patient (case #1) with a G2 lesion achieved complete remission after undergoing palliative chemotherapy (Sutent®, sunitinib malate, Pfizer) and lived for 110 months without recurrence. The second patient (case #3) with NEC developed hepatic metastasis 27 months after complete resection and received radiofrequency ablation and chemotherapy (etoposide and cisplatin). The patient died at 66 months and exhibited progression after recurrence. The third patient (case #14) with a GB tumor had MiNEN. He received adjuvant concurrent chemoradiation therapy (5-fluorouracil and leucovorin-based) after surgery but did not complete the scheduled course due to its complications. Nine months after surgery, tumors recurred in the liver, and partial liver resection was performed. The patient exhibited no evidence of recurrence for 12 months (Table 4).
In 2019, the grading and staging system for NENs was significantly changed. The first change was a new subset of well-differentiated NENs, particularly G3 NEN lesions, which are well-differentiated but have a high ki-67 index (> 20%) and mitotic count > 20 per 2 mm2. The second change was a change in the term from mixed adenoneuroendocrine carcinoma to MiNEN. MiNEN should comprise at least 30% of non-neuroendocrine components [11]. In our study, we had one patient with G1, five with G2, and five with pure poorly differentiated carcinoma (small-cell type, G3). We identified two patients with combined carcinoma, which made up < 30% of the tumor burden, and three patients with MiNEN. In each, the final category was poorly differentiated small-cell type carcinoma.
It is difficult to diagnose NENs preoperatively due to their low incidence. A nationwide study for NEN reported that 1.8% of all NENs originated from the biliary tract [12]. Several diagnostic imaging techniques, such as computed tomography (CT), magnetic resonance image (MRI), and positron emission tomography, have been used to evaluate patients with NENs, but each has some limitations, especially in cases of extrahepatic bile duct NENs [12]. Endoscopic ultrasound or endoscopic ultrasound-guided biopsy has been effective in some specific cases, such as in patients with tumors of AoV origin or those with direct liver invasion [13]. Even in patients with tumors of AoV origin, the biopsy confirmation rate for submucosal lesions is relatively low, ranging from 14% to 66%. Also, a definitive diagnosis using the mitotic count preoperatively is difficult due to its low availability and accuracy [12]. In our study, six patients were diagnosed with NENs preoperatively by biopsy; however, accurate grading was not possible.
NEN management is complex; hence, adequate staging of the lesion is needed for making a correct decision. Burns and Edil [14] demonstrated that complete resection of resectable locoregional NENs achieved good outcomes. However, no rational surgical strategy exists for several reasons, including the rarity of the disease, inability to predict progression, and limited understanding of the biology of the lesion or predictive prognostic factors [6]. In our study, two patients with GB and AoV lesions underwent hepatopancreatoduodenectomy. They remained recurrence-free for 153 months and 32 months, respectively. Our outcomes indicate that surgery for patients with locoregional NENs may improve the odds of survival if complete resection is possible.
The incidence rate of LN metastases is approximately 50% [15], resulting in recommendations with respect to the procedure of choice for NEN treatment, such as radical LN dissection. But LN metastases was not a significant factor for long-term survival. The American Joint Committee on Cancer TNM and European Neuroendocrine Tumor Society staging systems are limited in predicting the prognosis; thus an advanced stage does not predict a worse prognosis. However, surgical resection combined with regional LN dissection has been recommended for treatment and staging [16]. Nine patients had LN metastases in our study, and there was no significant difference in long-term survival.
The stage-for-stage prognosis of gut NENs is better than that for adenocarcinoma at similar sites. Poor prognostic factors include advanced age, incomplete surgical resection, tumor spread, and high-grade or poorly differentiated histology [12,17]. In our analysis, advanced age (> 65 years), mitotic count (> 20 per 2 mm2), and Ki67 > 20% had significant prognostic values for OS rates. DFS-related factors were advanced age (> 65 years) and mitotic count (> 20 per 2 mm2).
Interestingly, in contrast to AoV lesions, non-AoV lesions had pathologically different classifications according to the WHO guidelines. NENs are slow growing in nature, and their early detection during health screening or evaluation for other diseases may not be easy. In our study, patients with advanced age (> 65 years) were more frequently found in the non-AoV group than in the AoV group (62.5% and 12.5%, p = 0.039). High Ki-67 index (> 20%) and PNI were also more common in non-AoV patients than in AoV patients (100% vs. 25%; and 87.5% vs. 0%, respectively). One patient underwent R1 resection for a non-AoV lesion remaining in the adenocarcinoma portion of the remnant bile duct and died 12 months postoperatively.
Preoperative diagnosis of NENs remains difficult despite developments in imaging studies. Cholangiocarcinoma presents significantly similar characteristics and morphology as NENs when evaluated using various modalities, including ultrasound, CT, or MRI. Endoscopic ultrasound-guided biopsy is considered more useful in specific conditions [18]. However, preoperative biopsy cannot accurately determine whether the tumor is a NEN or NEC. In our study, preoperative biopsy confirmation was possible in only six patients and one patient in the AoV and non-AoV groups, respectively.
Chromogranin A is elevated in 90% of gut NENs and is associated with tumor burden and recurrence. Therefore, serum chromogranin A could be an effective biomarker for a more accurate preoperative diagnosis of NENs. However, it has a limitation of cost-effectiveness due to the rarity of extrahepatic biliary NENs [19].
Clear treatment guidelines for NEN have not yet been clearly established; however, complete surgical resection should be performed for long-term survival, similar to other hepatobiliary malignant tumors. According to our experience, aggressive adjuvant treatment can help to prevent recurrence of disease, such as NEC or MiNEN. But, the roles of adjuvant radiotherapy and chemotherapy in the management of NEC or MiNEN remain unclear. Traditional radiotherapy is generally ineffective for treating NENs [20]. Iwasa et al. [21] retrospectively examined the clinical data of 21 patients with unresectable or recurrent poorly differentiated NEC arising from the hepatobiliary tract and pancreas and who received combination chemotherapy with cisplatin and etoposide as the first-line treatment. Although no complete responses were obtained, three patients had partial responses, resulting in an overall response rate of 14%.
The limitations of studies on NENs are mostly caused by the rarity of the disease. In this study, we found several possible predictive factors for survival and disease recurrence; however, most of them could not be demonstrated accurately due to the small number of patients. For these reasons, large–scale multicenter studies are required.
In conclusion, AoV and non-AoV tumors had significant differences in the histologic grade, Ki67, and PNI. Patients with non-AoV tumors had more risk factors for survival and recurrence than those with AoV tumors. Early detection of tumors, adequate surgery, and aggressive adjuvant treatment for high-risk patients are important to achieve long-term survival and prevent the recurrence of extrahepatic biliary neuroendocrine tumors.
Notes
REFERENCES
1. Gustafsson BI, Kidd M, Modlin IM. 2008; Neuroendocrine tumors of the diffuse endocrine system. Curr Opin Oncol. 20:1–12. DOI: 10.1097/CCO.0b013e3282f1c595. PMID: 18043250.
2. Modlin IM, Sandor A. 1997; An analysis of 8305 cases of carcinoids tumors. Cancer. 79:813–829. DOI: 10.1002/(SICI)1097-0142(19970215)79:4<813::AID-CNCR19>3.0.CO;2-2.
3. Kim J, Lee WJ, Lee SH, Lee KB, Ryu JK, Kim YT, et al. 2011; Clinical features of 20 patients with curatively resected biliary neuroendocrine tumours. Dig Liver Dis. 43:965–970. DOI: 10.1016/j.dld.2011.07.010. PMID: 21856258.

4. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. 2020; The 2019 WHO Classification of tumours of the digestive system. Histopathology. 76:182–188. DOI: 10.1111/his.13975. PMID: 31433515. PMCID: PMC7003895.

5. de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, et al. 2017; Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms. Neuroendocrinology. 105:412–425. DOI: 10.1159/000475527. PMID: 28803232.

6. Eltawil KM, Gustafsson BI, Kidd M, Modlin IM. 2010; Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol. 44:687–695. DOI: 10.1097/MCG.0b013e3181d7a6d4. PMID: 20375728.
7. Nishime C, Ohnishi Y, Suemizu H, Tamaoki N, Kusumi T, Sato F, et al. 2008; In vivo chemotherapeutic profile of human gallbladder small cell carcinoma. Biomed Res. 29:251–256. DOI: 10.2220/biomedres.29.251. PMID: 18997440.

8. Jun SR, Lee JM, Han JK, Choi BI. 2006; High-grade neuroendocrine carcinomas of the gallbladder and bile duct: report of four cases with pathological correlation. J Comput Assist Tomogr. 30:604–609. DOI: 10.1097/00004728-200607000-00009. PMID: 16845291.
9. Lane JE, Walker AN, Ayers GW, Foster JL, Williams JT. 2002; Small-cell undifferentiated carcinoma of neuroendocrine type originating in the gallbladder. Curr Surg. 59:495–497. DOI: 10.1016/S0149-7944(02)00638-4. PMID: 15727797.

10. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Neuroendocrine tumors. [Online, 16 December, 2017]. National Comprehensive Cancer Network.
11. Klimstra D, Klöppel G, La Rosa S, Rindi G. 2019; Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board, ed. Digestive system tumours WHO classification of tumours. 5th ed. IARC,. 16–19.
12. Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, et al. Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: multicenter study. Cancer Res Treat. 2012; 44:157–165. DOI: 10.4143/crt.2012.44.3.157. PMID: 23091441. PMCID: PMC3467418.

13. Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. 2019; Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 54:19–32. DOI: 10.1007/s00535-018-1519-2. PMID: 30406288. PMCID: PMC6314985.

14. Burns WR, Edil BH. 2012; Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol. 13:24–34. DOI: 10.1007/s11864-011-0172-2. PMID: 22198808.

15. Zheng Z, Chen C, Li B, Liu H, Zhou L, Zhang H, et al. 2019; Biliary neuroendocrine neoplasms: clinical profiles, management, and analysis of prognostic factors. Front Oncol. 9:38. DOI: 10.3389/fonc.2019.00038. PMID: 30805307. PMCID: PMC6370735.

16. Parekh JR, Wang SC, Bergsland EK, Venook AP, Warren RS, Kim GE, et al. 2012; Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas. 41:840–844. DOI: 10.1097/MPA.0b013e31823cdaa0. PMID: 22781907.

17. Lee L, Ito T, Jensen RT. 2019; Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine neoplasm: recent advances and controversies. Expert Rev Anticancer Ther. 19:1028–1050. DOI: 10.1080/14737140.2019.1693893. PMID: 31738624. PMCID: PMC6923565.
18. Jayant M, Punia R, Kaushik R, Sharma R, Sachdev A, Nadkarni NK, et al. 2012; Neuroendocrine tumors of the ampulla of Vater: presentation, pathology and prognosis: presentation. JOP. 13:263–267.
19. Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. 2010; Chromogranin A-biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 17:2427–2443. DOI: 10.1245/s10434-010-1006-3. PMID: 20217257.

20. Modlin IM, Kidd M, Drozdov I, Siddique ZL, Gustafsson BI. 2008; Pharmacotherapy of neuroendocrine cancers. Expert Opin Pharmacother. 9:2617–2626. DOI: 10.1517/14656566.9.15.2617. PMID: 18803449.

21. Iwasa S, Morizane C, Okusaka T, Ueno H, Ikeda M, Kondo S, et al. 2010; Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 40:313–318. DOI: 10.1093/jjco/hyp173. PMID: 20047862.

Fig. 1
Overall and disease-free survival rates of patients with neuroendocrine tumors. (A) The 1-, 3-, and 5-year overall survival rates were 87.5%, 81.3%, and 72.2%, respectively. (B) The 1-, 3-, and 5-year disease-free survival rates were 68.8%, 62.5%, and 62.5%, respectively.
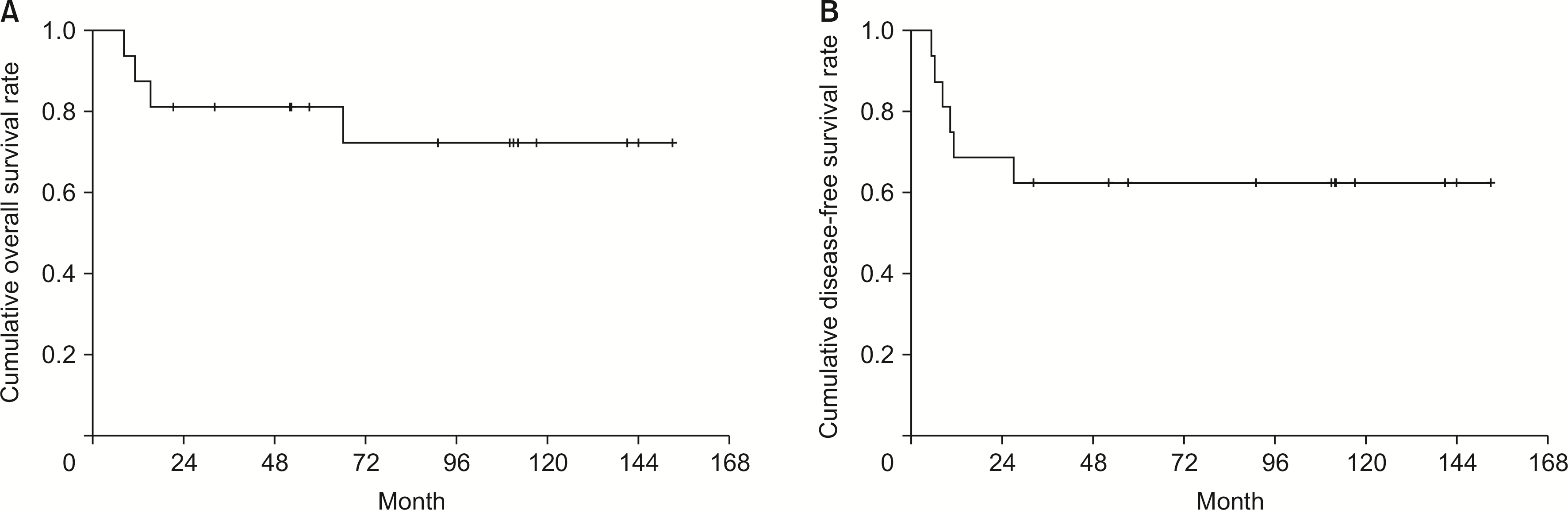
Fig. 2
The survival and DFS comparison of the AoV and non-AoV groups. (A) The 1-, 3-, and 5-year postoperative OS rates: 87.5%, 87.5%, and 72.9%; and 87.5%, 75.0%, and 75.0%, p = 0.782, respectively. (B) The 1-, 3-, and 5-year postoperative DFS rates: 75.0%, 62.5%, and 62.5%; and 62.5%, 62.5%, and 62.5%, p = 0.869, respectively. DFS, disease-free survival; AoV, ampulla of Vater; OS, overall survival.
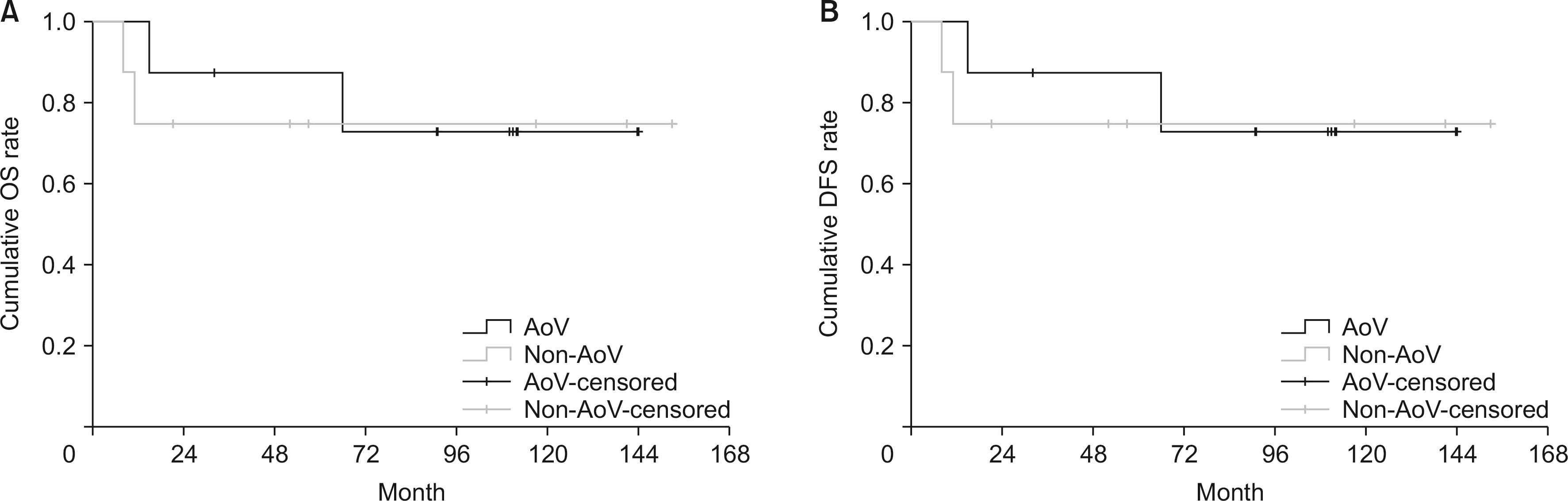
Table 1
Classification and grading criteria for NENs of the gastrointestinal tract and hepatopancreatobiliary organs
Cited from the article of Nagtegaal et al. Histopathology 2020;76:182-188 [4].
Table 2
Comparison of the clinicopathologic characteristics of patients with NENs
| Variables | Total | AoV (n = 8) | Non-AoV (n = 8) | p |
|---|---|---|---|---|
| Age (yr) | 0.039 | |||
| ≤ 65 | 10 (62.5) | 7 (87.5) | 3 (37.5) | |
| > 65 | 6 (37.5) | 1 (12.5) | 5 (62.5) | |
| Sex | 0.614 | |||
| Male | 7 (43.8) | 4 (50.0) | 3 (37.5) | |
| Female | 9 (56.2) | 4 (50.0) | 5 (62.5) | |
| Comorbidities | 0.590 | |||
| Yes | 5 (31.3) | 3 (37.5) | 2 (25.0) | |
| No | 11 (68.7) | 5 (62.5) | 6 (75.0) | |
| Symptoms | > 0.999 | |||
| Yes | 6 (37.5) | 3 (37.5) | 3 (37.5) | |
| No | 10 (62.5) | 5 (62.5) | 5 (62.5) | |
| Mitosis (per 2 mm2) | > 0.999 | |||
| ≤ 20 | 12 (75.0) | 6 (75.0) | 6 (75.0) | |
| > 20 | 4 (25.0) | 2 (25.0) | 2 (25.0) | |
| Ki-67 (%) | 0.002 | |||
| ≤ 20 | 6 (37.5) | 6 (75.0) | 0 (0) | |
| > 20 | 10 (62.5) | 2 (25.0) | 8 (100) | |
| Histologic grade | 0.022 | |||
| G1 | 1 (6.2) | 1 (12.5) | 0 (0) | |
| G2 | 5 (31.2) | 5 (62.5) | 0 (0) | |
| G3 | 0 (0) | 0 (0) | 0 (0) | |
| NEC | 5 (31.2) | 1 (12.5) | 4 (50.0) | |
| MiNEN | 5 (31.2) | 1 (12.5) | 4 (50.0) | |
| T statusa) | > 0.999 | |||
| ≤ T2 | 10 (62.5) | 5 (62.5) | 5 (62.5) | |
| > T2 | 6 (37.5) | 3 (37.5) | 3 (37.5) | |
| LN metastasis | 0.614 | |||
| Present | 9 (56.2) | 5 (62.5) | 4 (50.0) | |
| Absent | 7 (43.8%) | 3 (37.5) | 4 (50.0) | |
| Harvested LNs (median, range) | ||||
| Total LNs | 17 (2–86) | 18.5 (10–50) | 16 (2–86) | 0.680 |
| Metastatic LNs | 1 (0–4) | 1 (0–4) | 0.5 (0–1) | 0.334 |
| LVI | 0.590 | |||
| Present | 11 (68.7) | 5 (62.5) | 6 (75.0) | |
| Absent | 5 (31.3) | 3 (37.5) | 2 (25.0) | |
| PNI | < 0.001 | |||
| Present | 7 (43.8) | 0 (0) | 7 (87.5) | |
| Absent | 9 (56.2) | 8 (100) | 1 (12.5) | |
| Adjuvant chemotherapy | 0.590 | |||
| Yes | 11 (68.8) | 6 (75.5) | 5 (62.5) | |
| No | 5 (31.2) | 2 (25.0) | 3 (37.5) |
Table 3
Univariate and multivariate analyses of overall survival and disease-free survival rates
| Variable | Overall survival | Disease free survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p | HR | 95% CI | p | p | HR | 95% CI | p | |
| Age > 65 yr | 0.018 | 0.491 | 0.018–13.08 | 0.671 | 0.033 | 0.525 | 0.020–13.56 | 0.698 |
| Sex (female) | 0.437 | - | - | - | 0.489 | - | - | - |
| Tumor location (non-AoV) | 0.717 | - | - | - | 0.797 | - | - | - |
| Mitosis > 20 (per 2 mm2) | 0.009 | 17.48 | 1.269–240.79 | 0.033 | 0.010 | 17.66 | 1.267–246.06 | 0.033 |
| Ki67 > 20 (%) | 0.044 | 0.000 | - | 0.975 | 0.059 | - | - | - |
| Histologic grade (G1/G2 vs. NEC/MiNEN) | 0.065 | - | - | - | 0.081 | - | - | - |
| T status (T3, T4)a) | 0.069 | - | - | - | 0.068 | - | - | - |
| LN metastasis | 0.456 | - | - | - | 0.446 | - | - | - |
| LVI | 0.657 | - | - | - | 0.672 | - | - | - |
| PNI | 0.467 | - | - | - | 0.548 | - | - | - |
| Adjuvant chemotherapy | 0.886 | - | - | - | 0.956 | - | - | - |
Table 4
Clinicopathologic characteristics and outcomes of patients with NENs
| Case | Sex/age (yr) | Cormorbidity/symptom | Tumor location | Histologic grade | Mitotic count | Ki-67 | Operative method | T statusa) | LN status | LVI | PNI | Adjuvant treatment | Disease recurrence | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | M/62 | +/– | AoV | NET, G2 | 1 | 5 | PD | 2 | 0 (0/50) | – | – | + | + | Ned |
| #2 | M/36 | –/– | AoV | NET, G2 | 4 | 4 | PD | 3 | 1 (4/20) | + | – | + | – | Ned |
| #3 | M/59 | +/jaundice | AoV | MiNEN | 102 | 70 | PPPD | 3 | 1 (1/20) | + | – | + | + | Expired |
| #4 | F/58 | +/– | AoV | NET, G2 | 5 | 3 | PPPD | 2 | 1 (1/17) | – | – | + | – | NED |
| #5 | F/47 | –/– | AoV | NET, G1 | 1 | 1 | PPPD | 2 | 0 (0/12) | + | – | + | – | NED |
| #6 | M/43 | +/pain | AoV | NET, G2 | 2 | 2.2 | HPD | 2 | 0 (0/10) | + | – | NA | – | NED |
| #7 | F/74 | +/dyspepsia | AoV | NEC | 250 | 80 | PD | 3 | 1 (1/15) | + | – | + | + | Expired |
| #8 | F/38 | –/– | AoV | NET, G2 | 1 | 4.7 | PPPD | 2 | 1 (1/23) | – | – | NA | – | NED |
| #9 | F/49 | –/– | GB | NEC | 2 | 30 | RC | 2 | 0 (0/15) | + | + | + | – | NED |
| #10 | M/66 | +/– | GB | NEC | 8 | 25 | RC | 2 | 1 (1/2) | – | + | NA | – | NED |
| #11 | F/74 | +/– | GB | NEC | 15 | 30 | RC | 3 | 1 (1/49) | + | + | NA | + | Expired |
| #12 | F/71 | +/– | GB | MiNEN | 52 | 70 | RC | 2 | 0 (0/6) | + | + | NA | – | NED |
| #13 | F/44 | –/pain | GB | MiNEN | 0 | 60 | HPD | 4 | 1 (1/86) | + | – | + | – | NED |
| #14 | M/74 | +/– | GB | MiNEN | 0 | 90 | RC | 3 | 1 (1/17) | + | + | + | + | NED |
| #15 | M/64 | +/jaundice | EBD | NEC | 20 | 60 | PPPD | 2 | 0 (0/21) | + | + | + | – | NED |
| #16 | F/67 | +/pain | EBD | MiNEN | 23 | 90 | rBDR | 2 | 0 (0/8) | – | + | + | + | Expired |
NEN, neuroendocrine neoplasm; AoV, ampulla of Vater; GB, gallbladder; EBD, extrahepaticbile duct; NET, neuroendocrine tumor; MiNEN, mixed neuroendocrine-non-neuroendocrine neoplasm; NEC, neuroendocrine carcinoma; PD, pancreaticoduodenectomy; PPPD, pyrolus preserving pancreaticoduodenectomy; RC, radical cholecystectomy; HPD, hepatopancreaticoduodenectomy; rBDR, radical bile duct resection; LN, lymph node; LVI, lymphovascular invasion; PNI, perineural invasion; NA, not available.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download