Abstract
The concept of research integrity (RI) refers to a set of moral and ethical standards that serve as the foundation for the execution of research activities. Integrity in research is the incorporation of principles of honesty, transparency, and respect for ethical standards and norms throughout all stages of the research endeavor, encompassing study design, data collecting, analysis, reporting, and publishing. The preservation of RI is of utmost importance to uphold the credibility and amplify the influence of scientific research while also preventing and dealing with instances of scientific misconduct. Researchers, institutions, journals, and readers share responsibilities for preserving RI. Researchers must adhere to the highest ethical standards. Institutions have a role in establishing an atmosphere that supports integrity ideals while also providing useful guidance, instruction, and assistance to researchers. Editors and reviewers act as protectors, upholding quality and ethical standards in the dissemination of research results through publishing. Readers play a key role in the detection and reporting of fraudulent activity by critically evaluating content. The struggle against scientific misconduct has multiple dimensions and is continuous. It requires a collaborative effort and adherence to the principles of honesty, transparency, and rigorous science. By supporting a culture of RI, the scientific community may preserve its core principles and continue to contribute appropriately to society’s well-being. It not only aids present research but also lays the foundation for future scientific advancements.
The importance of ‘Research Integrity’ (RI) cannot be underestimated. RI is regarded as a mechanism that serves the dual purpose of safeguarding the professional careers and reputation of researchers while upholding societal confidence in scientists and research. RI plays a significant role in fostering economic and social advancements. It generates trust and benefits all stakeholders, including researchers, individuals, research funders, and governmental authorities responsible for science policy. RI is at the heart of the research process. It allows scientists to trust one another and the data results, and it serves as the foundation for the general public’s trust in research evidence, results, and scientists. RI protects patients and the public from the negative consequences of misleading and erroneous data.12
Scientific misconduct is defined as “intentional falsification of scientific data by presentation of fraudulent or incomplete or uncorroborated findings as scientific fact.”3 Misconduct can be associated with all activities involving humans, and given the internationalization of scientific research, scientific misconduct is thus a worldwide concern. Although the majority of research on scientific misconduct has been conducted in high-income countries, limited data from low- and middle-income countries suggest that research misconduct is also prevalent in these countries.4
Historically, RI has concentrated on addressing three core issues about scientific research5:
‘Fabrication’ refers to the act of making up data, conclusions, and scientific details that were not obtained or noticed during the examination process.
‘Falsification’ is changing or manipulating data, outcomes, or other research-related information into a format distinct from its original version.
‘Plagiarism’ refers to the unauthorized utilization of intellectual property belonging to others, encompassing a wide range of materials such as academic papers, research methodologies, visual representations, and conceptual frameworks.67
As is evident from the preceding definition of scientific misconduct, the scope is limited. Consequently, it does not adequately reflect the variety of RI challenges. Other improper conduct, such as undisclosed conflicts of interest, violations of research policies, concerns with informed consent forms, violations of ethics committee regulations, and fraudulent peer review, can jeopardize the reliability of research processes and outcomes. They are also likely to have a significant effect on research efforts. These acts are known as questionable research practices. Ignoring and hiding specific research outcomes and highlighting the most interesting ones can also be considered.589
The main objective of this overview is to provide a general perspective on RI and scientific misconduct. Secondly, we touched upon the components of the academic sphere that have responsibilities in the context of scientific misconduct. In addition, we emphasized scientific misconduct prevention strategies. Finally, the responsibilities of journal editors and reviewers in providing RI were summarized.
Gasparyan et al.10 presented recommendations for comprehensive and systematic search strategies. Based on these recommendations, we designed our search strategy. We compiled a list of keyword combinations involving ‘Scientific Misconduct,’ ‘Research Misconduct,’ ‘Ethics in Publishing,’ ‘Scientific Fraud,’ and ‘Scientific Dishonesty.’ When choosing our search queries, we considered the presence of Medical Subject Headings terms. We searched through MEDLINE/PubMed, Scopus, Web of Science, and Directory of Open Access Journals. All article categories were included in the searches. Publications inconsistent with our objectives were excluded. No time constraints or intervals were set when we created our search strategy.
The scientific community has viewed self-regulation as the basis of RI, including the concepts of rights and responsibilities. The onus of self-regulation is with individual researchers, who are responsible for upholding the accuracy and reliability of their works and outcomes. This procedure also involves pertinent organizations, such as research institutions and financing departments, which have organizational responsibility for their employees and members. Researchers must assume a sense of responsibility in promoting the progress of scientific knowledge while adhering to the highest standards of integrity.11
Since scientific papers play an essential role in clinical practice and academic endeavors, the principles of appropriate authorship should be considered.12 The growing importance of authorship within academic spheres has been accompanied with multiple instances of unethical conduct. Ghostwriters, guest authors, and gift authors are frequently debated instances of inappropriate authorship. Ghostwriters are individuals who contribute substantially to scientific documents and avoid listing their names in the author bylines. One of the reasons ghostwriting is a conflict of interest is because it excludes transparent authorship.13 In contrast, guest or gift authors are those who are listed in the author bylines without substantial contributorships throughout the entire research process, starting from the hypotheses formulation to writing and publishing.14
Researchers are responsible for generating ethical hypotheses, adhering to the regulations of ethics committees, obtaining written informed consents, collecting, analyzing, and interpreting data, correctly listing co-authors, contributing to revisions, choosing target journals, and taking full responsibility for all aspects of their works. Researchers must conduct this entire procedure ethically and by self-regulating.15
Research supervisors should arrange comprehensive discussions regarding scientific misconduct and RI with their students and trainees. If a student or inexperienced researcher encounters challenges in an experiment, the mentors should guide for comprehending the underlying causes of the issue and suggesting appropriate steps for corrections. The mentor may also recall their own achievements and failures, serving as a role model. Errors committed within the scientific realm should not be regarded as mere challenges but rather as valuable occasions for teaching students the fundamental principles of scientific practice. Inexperienced researchers may inadvertently make errors and overlook essential details at the early stages of their research projects, potentially leading to misconduct. Mentors must maintain continuous oversight over their researchers. The occurrence of errors should not be regarded as a source of shame for researchers but rather as a chance for advancing research skills.1617
It is crucial for supervisors and mentors to actively execute their responsibilities to facilitate the growth of their mentees as honest and ethical researchers, thus enhancing the trustworthiness and integrity of the scientific field.
Numerous institutions are currently undertaking various initiatives to mitigate the issue of research misconduct. Academic institutions often monitor scholarly activities that may violate the basic principles of ethical research, writing, and publishing. Researchers committing misconduct often face consequences. Nonetheless, one of the crucial mechanisms is to prevent scientific misconduct rather than punish unethical individuals. Drafting and enforcing protocols and guidelines for ethical practice are essential steps for upholding RI.18
Nurturing an atmosphere of academic freedom is essential for science growth. However, creating mechanisms for preventing scientific misconduct and safeguarding RI is equally important. Research institutions worldwide should reach a consensus on methods aimed at regulating research activities and developing recommendations for promoting adherence to sound scientific and research practices. Universities and research institutions may adapt these recommendations to their local circumstances. Research institutions should monitor research projects, peer review evaluations, and dissemination of research results. Furthermore, institutions should protect research participants’ rights and uphold standards of data recording. To effectively deliver these services, it would be beneficial to establish RI departments.1920
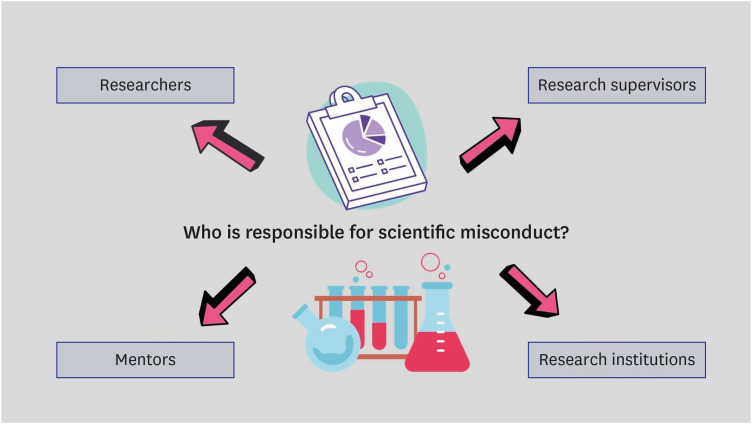
Due to the significance of the corporate image, some individuals and organizations may endeavor to downplay the disclosure of unfavorable information, thereby impeding the transparent dissemination of research results via news outlets and online platforms. Institutional authorities may be inclined to disregard or downplay charges of potential research misconduct to safeguard researchers’ financial interests. The authorities may lack comprehension of the significance of assessing research misconduct and may have limited capabilities to undertake an investigation effectively.21 Key elements responsible for scientific misconduct are visualized in Fig. 1.
Various solutions are available for mitigating instances of scientific misconduct. There is no one-size-fits-all answer to this big issue. The following section presents an overview of potential approaches.2122
The initial step in mitigating scientific misconduct involves establishing an information program and educating about acceptable clinical practices and research standards. The provision of education should be extended to encompass all facets of academic activities. A considerable proportion of errors may be regarded as unintentional. These honest errors stem from misconceptions in methodology, limited expertise, and insufficient education.23 Education is a prerequisite for preventing these errors, which may be overlooked by reviewers and editors. Suggestions on ethical publishing norms should be incorporated in educational programs. An analysis revealed a notable occurrence of scientific misconduct and violation of accepted publishing norms among young undergraduates and those residing in non-English-speaking nations.2425 These particular groups should be the focus of educational efforts.
The ethics committee is an autonomous entity comprising experts with knowledge and skills in scientific research and related activities. Its primary objective is to safeguard the rights of research participants and ensure that research activities follow ethical norms. The approval of research activities is dependent on a comprehensive evaluation of research protocols by ethics committees affiliated to academic institutions or hospitals.26 Even after obtaining the ethics approval/waivers, some institutions and universities fail to monitor compliance with specified protocols and standards. Hence, it is essential to establish mechanisms that offer monitoring of the entire process after obtaining the ethics considerations by ethics committees.27
Reviewers offer their services voluntarily and are chosen by editors based on their established academic reputation and expertise in a particular field. The reviewers play a crucial role not only in the rejection of publications that fail to meet the required scientific standards but also in identifying and reporting scientific misconduct. Topical training programs can be arranged for them, accompanied by various rewarding mechanisms, to enhance the efficacy of the reviewing process. Considering peer-review activities as essential components of scholars’ academic achievements should be a part of complex measures for upholding writing, editing, and publishing standards.2829
Research institutions should establish mechanisms that allow whistleblowers to expose unethical conduct without fear of retaliation. It is critical to protect individuals who expose scientific misconduct to maintain RI. Reporting of scientific misconduct may become widespread, with institutions and regulatory agencies strengthening whistleblower protection. Research managers should ensure that reports on misconduct are properly dealt with and not discarded.3031
Inexperienced researchers should be educated to avoid being trapped by predatory journals. They should be aware of unethical and poor open access journals that damage their authors’ academic reputations. Predatory journals do not actively employ plagiarism detection procedures and lack robust peer review evaluations. The objective should be to raise researchers’ awareness of the predatory publishing characteristics and mechanisms to publish in reliable and ethical journals. It is crucial to caution researchers who publish in predatory journals and inform them about the dire consequences of the short-cuts in the publishing enterprise.3233
Open science is a global initiative that seeks to enhance the transparency, partnership, and accessibility of the scientific process.34 The implementation of open science approaches yields numerous advantages and can uphold standards and implications of reliable research.35
The sharing of research data is regarded as a crucial aspect of open science. Enabling open access to research data facilitates open post-publication evaluation. The utilization of open data also facilitates the verification of research outcomes and the formulation of innovative research strategies. The promotion of openness enhances the trust in the accuracy and reliability of data. The advent of digitalization has brought about significant transformations in the publishing enterprise. The Open Access global initiative has already positively impacted the visibility and accessibility of scientific articles.3637 Strategies to avoid scientific misconduct are summarized in Fig. 2.
Scientific journals serve a crucial role in the research process as they facilitate the distribution of data. Hence, editors need to safeguard the integrity of the scientific papers they disseminate. The frequency of prominent retractions, particularly in esteemed scientific journals, reveals the shortcomings of traditional prepublication peer review in identifying fraudulent or unethical studies.3839 On the other hand, these retractions emphasize the effectiveness of the publishing enterprise. Editors fulfill their duties, act responsibly, and retract inaccurate and misleading articles.40 Journal editors may be hesitant to initiate the retraction procedure. One of the reasons for the hesitance is the editors’ unjustified fear of damaging their journal’s reputation.41 To prevent disseminating misleading information, editors should be updated on guidelines for retracting fraudulent, erroneous, and misleading reports.42
Journal editors can proactively detect and reject potentially retractable reports at the in-house and external evaluation stages. They can be influential instructors, establishing good research strategies for current and prospective authors. Journal instructions and ethics statements should not only advise authors but also educate reviewers and editors on their responsibilities.43
Journals employ several strategies to minimize and expose instances of scientific misconduct. A prime example is requesting the authors to confirm that their submissions are authentic, previously unpublished, adherent to ethical authorship regulations, and open data sharing opportunities.
Most journals currently employ plagiarism detection software to identify instances of copying and otherwise unlawful writing, reusing graphics, misappropriating ideas, and failing to properly reference.44 A growing number of journals request their authors to list all contributors’ identifiers and disclose external writing and editing support.
Scientific journals should transparently display their editorial and publishing policies. Merely providing links to global editorial guidelines without related interpretations and definitions can be viewed as poor editorial practice. In today’s world, websites have become repositories of journal rules and regulations, serving as crucial resources for authors. Implementing enhanced transparency in establishing authorship policy has been suggested as a means to mitigate misinterpretation and prevent inappropriate attribution of authorship.45
The editorial staff cannot assess the scientific methodologies or the raw data in each and every submitted article. Nevertheless, when faced with uncertainty, they can adopt an effective strategy by requesting access to the raw data and seeking the expertise of experienced statisticians to verify the results.4647
The role of readers in identifying and addressing scientific misconduct is essential as they serve a crucial role in exerting caution and critical analysis. Readers can check the references provided in the article. Misleading, erroneous, or non-existent references may point to misconduct.48 Readers may carefully analyze data and graphical materials. If inconsistencies contradict conclusions, journal editors can be contacted, and raw data can be requested.49 The active engagement of readers in online platforms and the involvement of post-publication peer review processes have the potential to make a valuable contribution to this overall effort. Inconsistencies between formulated hypotheses, research questions, and employed research designs can also be detected by attentive readers. Overall, readers should keep up with the latest trends in research ethics, scientific misconduct, and retractions.
Individuals, institutions, and journals should all take particular steps to deal with and prevent scientific misconduct. To summarize the recommendations mentioned in the previous sections:
Researchers should consistently educate themselves regarding ethical principles and standards within their respective academic disciplines. It is imperative to maintain a state of being well-informed regarding the continuous development of ethical considerations. Strict data management practices should be put in place. Experiment, data collecting, and analysis records should be preserved on a regular and transparent basis. Researchers should communicate openly with their peers, mentors, and colleagues. Constructive criticism and peer review can assure research quality and integrity.
Institutions should provide thorough ethics training programs for both researchers and staff members. It is imperative that these programs be made compulsory and implemented in a repetitious manner. Institutions should establish confidential reporting mechanisms to document individual abuse cases and foster an atmosphere of trust. The ethics committee system should be developed as much as possible. It is necessary to develop an institutional culture that values RI by rewarding and recognizing ethical behavior.
Journals should keep a strict peer-review mechanism in place to check papers for ethical and methodological concerns. It is essential to establish precise guidelines for retractions and corrections. The retraction process needs to be transparent. Journals should make every effort to detect plagiarism. Editorial independence should be preserved, and editors should be protected from excessive influence that could jeopardize RI.
The preservation of RI is of utmost importance in the pursuit of expanding the boundaries of knowledge and upholding the credibility of scientific investigations. Scientific misconduct not only undermines the basis of trust within the academic community but also poses significant obstacles to the development of scientific understanding.
RI is a collective responsibility shared by researchers, institutions, journals, and readers. Researchers must uphold the utmost ethical standards throughout the entire research process, encompassing the formulation of experimental designs as well as the dissemination of results. Institutions have a role in cultivating an environment that upholds principles of integrity. Editors and reviewers serve as protectors, responsible for maintaining the publishing standards. Readers play a crucial part in the identification and reporting of fraudulent activities by engaging in a critical evaluation of content.
The battle against scientific misconduct is multidirectional and continuous. It takes a team effort as well as dedication to the principles of honesty, transparency, and rigorous science. The scientific community may preserve its essential ideals and continue to contribute effectively to the well-being of society by encouraging a culture of RI.
Notes
Author Contributions:
Conceptualization: Zhaksylyk A, Zimba O, Kocyigit BF.
Data curation: Zhaksylyk A, Zimba O, Yessirkepov M, Kocyigit BF.
Formal analysis: Kocyigit BF.
Investigation: Zhaksylyk A, Zimba O, Yessirkepov M, Kocyigit BF.
Methodology: Zhaksylyk A, Zimba O.
Software: Kocyigit BF.
Writing - review & editing: Zhaksylyk A, Zimba O, Yessirkepov M, Kocyigit BF.
References
1. Science Europe. Seven reasons to care about integrity in research. Updated 2015. Accessed October 2, 2023.
https://www.scienceeurope.org/media/42sphgqt/20150617_seven-reasons_web2_final.pdf
.
2. Dinis-Oliveira RJ. COVID-19 research: pandemic versus “paperdemic”, integrity, values and risks of the “speed science”. Forensic Sci Rev. 2020; 5(2):174–187.

3. National Library of Medicine. Medical Subject Headings (MeSH) dictionary: ethics. Updated 2023. Accessed October 2, 2023.
https://www.ncbi.nlm.nih.gov/mesh/68015871
.
4. Ana J, Koehlmoos T, Smith R, Yan LL. Research misconduct in low- and middle-income countries. PLoS Med. 2013; 10(3):e1001315. PMID: 23555197.

5. Bouter L. What research institutions can do to foster research integrity. Sci Eng Ethics. 2020; 26(4):2363–2369. PMID: 31965429.

6. Rodrigues F, Gupta P, Khan AP, Chatterjee T, Sandhu NK, Gupta L. The cultural context of plagiarism and research misconduct in the Asian region. J Korean Med Sci. 2023; 38(12):e88. PMID: 36974397.

7. Kocyigit BF, Akyol A. Analysis of retracted publications in the biomedical literature from Turkey. J Korean Med Sci. 2022; 37(18):e142. PMID: 35535370.

8. Haven TL, Tijdink JK, Pasman HR, Widdershoven G, Ter Riet G, Bouter LM. Researchers’ perceptions of research misbehaviours: a mixed methods study among academic researchers in Amsterdam. Res Integr Peer Rev. 2019; 4(1):25. PMID: 31819806.

9. Rivera H. Fake peer review and inappropriate authorship are real evils. J Korean Med Sci. 2018; 34(2):e6. PMID: 30636943.
10. Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011; 31(11):1409–1417. PMID: 21800117.

11. Satalkar P, Shaw D. How do researchers acquire and develop notions of research integrity? A qualitative study among biomedical researchers in Switzerland. BMC Med Ethics. 2019; 20(1):72. PMID: 31619226.

12. Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010; 144(1):1–2.

13. Aliukonis V, Poškutė M, Gefenas E. Perish or publish dilemma: challenges to responsible authorship. Medicina (Kaunas). 2020; 56(3):123. PMID: 32178434.

14. Singhal S, Kalra BS. Publication ethics: role and responsibility of authors. Indian J Gastroenterol. 2021; 40(1):65–71. PMID: 33481172.

15. Sanjari M, Bahramnezhad F, Fomani FK, Shoghi M, Cheraghi MA. Ethical challenges of researchers in qualitative studies: the necessity to develop a specific guideline. J Med Ethics Hist Med. 2014; 7:14. PMID: 25512833.
16. Resnik DB, Shamoo AE. Reproducibility and research integrity. Account Res. 2017; 24(2):116–123. PMID: 27820655.

17. Silver JK, Gavini N. The push-pull mentoring model: ensuring the success of mentors and mentees. J Med Internet Res. 2023; 25:e48037. PMID: 37227764.

18. Research integrity is much more than misconduct. Nature. 2019; 570(7759):5.
19. Olesen A, Amin L, Mahadi Z. Unethical authorship practices: a qualitative study in Malaysian higher education institutions. Developing World Bioeth. 2018; 18(3):271–278.

20. Martinson BC, Thrush CR, Lauren Crain A. Development and validation of the Survey of Organizational Research Climate (SORC). Sci Eng Ethics. 2013; 19(3):813–834. PMID: 23096775.

21. Titus SL, Wells JA, Rhoades LJ. Repairing research integrity. Nature. 2008; 453(7198):980–982. PMID: 18563131.

22. Cogan E. Preventing fraud in biomedical research. Front Cardiovasc Med. 2022; 9:932138. PMID: 36093176.

23. Hosseini M, Hilhorst M, de Beaufort I, Fanelli D. Doing the right thing: a qualitative investigation of retractions due to unintentional error. Sci Eng Ethics. 2018; 24(1):189–206. PMID: 28321689.

24. Gasparyan AY, Nurmashev B, Seksenbayev B, Trukhachev VI, Kostyukova EI, Kitas GD. Plagiarism in the context of education and evolving detection strategies. J Korean Med Sci. 2017; 32(8):1220–1227. PMID: 28665055.

25. Gupta L, Tariq J, Yessirkepov M, Zimba O, Misra DP, Agarwal V, et al. Plagiarism in non-anglophone countries: a cross-sectional survey of researchers and journal editors. J Korean Med Sci. 2021; 36(39):e247. PMID: 34636502.

26. Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M. Ethics committees: structure, roles, and issues. J Korean Med Sci. 2023; 38(25):e198. PMID: 37365729.

27. Pich J, Carné X, Arnaiz JA, Gómez B, Trilla A, Rodés J. Role of a research ethics committee in follow-up and publication of results. Lancet. 2003; 361(9362):1015–1016. PMID: 12660062.

28. Gasparyan AY, Gerasimov AN, Voronov AA, Kitas GD. Rewarding peer reviewers: maintaining the integrity of science communication. J Korean Med Sci. 2015; 30(4):360–364. PMID: 25829801.

29. Barroga E. Innovative strategies for peer review. J Korean Med Sci. 2020; 35(20):e138. PMID: 32449322.

30. Bouter LM, Hendrix S. Both whistleblowers and the scientists they accuse are vulnerable and deserve protection. Account Res. 2017; 24(6):359–366. PMID: 28481674.

31. Mechtenberg L, Muehlheusser G, Roider A. Whistleblower protection: theory and experimental evidence. Eur Econ Rev. 2020; 126:103447.

32. Johal J, Ward R, Gielecki J, Walocha J, Natsis K, Tubbs RS, et al. Beware of the predatory science journal: a potential threat to the integrity of medical research. Clin Anat. 2017; 30(6):767–773. PMID: 28509358.

33. Beall J. Dangerous predatory publishers threaten medical research. J Korean Med Sci. 2016; 31(10):1511–1513. PMID: 27550476.

34. Allen C, Mehler DM. Open science challenges, benefits and tips in early career and beyond. PLoS Biol. 2019; 17(5):e3000246. PMID: 31042704.

35. Errington TM, Iorns E, Gunn W, Tan FE, Lomax J, Nosek BA. An open investigation of the reproducibility of cancer biology research. Elife. 2014; 3:e04333. PMID: 25490932.

36. Gasparyan AY, Yessirkepov M, Voronov AA, Koroleva AM, Kitas GD. Comprehensive approach to open access publishing: platforms and tools. J Korean Med Sci. 2019; 34(27):e184. PMID: 31293109.

37. Huston P, Edge VL, Bernier E. Reaping the benefits of Open Data in public health. Can Commun Dis Rep. 2019; 45(11):252–256. PMID: 31647060.

38. Wager E, Jefferson T. Shortcomings of peer review in biomedical journals. Learn Publ. 2001; 14(4):257–263.

39. Peh WC. Peer review: concepts, variants and controversies. Singapore Med J. 2022; 63(2):55–60. PMID: 34602311.

40. Bosch X. Improving biomedical journals’ ethical policies: the case of research misconduct. J Med Ethics. 2014; 40(9):644–646. PMID: 24505117.

41. Williams P, Wager E. Exploring why and how journal editors retract articles: findings from a qualitative study. Sci Eng Ethics. 2013; 19(1):1–11. PMID: 21761244.

42. Gasparyan AY, Yessirkepov M, Voronov AA, Koroleva AM, Kitas GD. Updated editorial guidance for quality and reliability of research output. J Korean Med Sci. 2018; 33(35):e247. PMID: 30140192.

43. Wager E. How journals can prevent, detect and respond to misconduct. Notf Rettmed. 2011; 14(8):613–615.

44. Memon AR. Similarity and plagiarism in scholarly journal submissions: bringing clarity to the concept for authors, reviewers and editors. J Korean Med Sci. 2020; 35(27):e217. PMID: 32657084.

45. Bosch X, Pericas JM, Hernández C, Torrents A. A comparison of authorship policies at top-ranked peer-reviewed biomedical journals. Arch Intern Med. 2012; 172(1):70–72. PMID: 22232152.

46. Al-Marzouki S, Evans S, Marshall T, Roberts I. Are these data real? Statistical methods for the detection of data fabrication in clinical trials. BMJ. 2005; 331(7511):267–270. PMID: 16052019.

47. Miyakawa T. No raw data, no science: another possible source of the reproducibility crisis. Mol Brain. 2020; 13(1):24. PMID: 32079532.

48. Gøtzsche PC. Citation bias: questionable research practice or scientific misconduct? J R Soc Med. 2022; 115(1):31–35. PMID: 35105192.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download