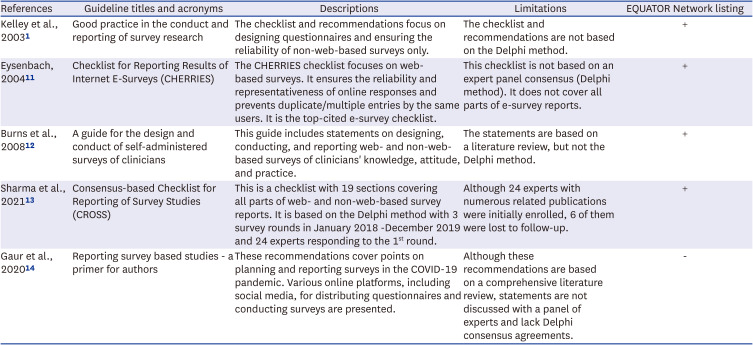
1. Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003; 15(3):261–266. PMID:
12803354.

2. Arafa AE, Anzengruber F, Mostafa AM, Navarini AA. Perspectives of online surveys in dermatology. J Eur Acad Dermatol Venereol. 2019; 33(3):511–520. PMID:
30317674.
3. Jones S, Murphy F, Edwards M, James J. Using online questionnaires to conduct nursing research. Nurs Times. 2008; 104(47):66–69.
4. Phillips AW, Friedman BT, Utrankar A, Ta AQ, Reddy ST, Durning SJ. Surveys of health professions trainees: prevalence, response rates, and predictive factors to guide researchers. Acad Med. 2017; 92(2):222–228. PMID:
27532869.

5. Latour JM, Tume LN. How to do and report survey studies robustly: a helpful mnemonic SURVEY. Nurs Crit Care. 2021; 26(5):313–314. PMID:
34053156.
6. Regmi PR, Waithaka E, Paudyal A, Simkhada P, van Teijlingen E. Guide to the design and application of online questionnaire surveys. Nepal J Epidemiol. 2016; 6(4):640–644. PMID:
28804676.

7. Evans JR, Mathur A. The value of online surveys. Internet Res. 2005; 15(2):195–219.

8. Li AH, Thomas SM, Farag A, Duffett M, Garg AX, Naylor KL. Quality of survey reporting in nephrology journals: a methodologic review. Clin J Am Soc Nephrol. 2014; 9(12):2089–2094. PMID:
25267553.
9. Pagano MB, Dunbar NM, Tinmouth A, Apelseth TO, Lozano M, Cohn CS, et al. A methodological review of the quality of reporting of surveys in transfusion medicine. Transfusion. 2018; 58(11):2720–2727. PMID:
30260483.

10. Shankar PR, Maturen KE. Survey research reporting in radiology publications: a review of 2017 to 2018. J Am Coll Radiol. 2019; 16(10):1378–1384. PMID:
31585659.

11. Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004; 6(3):e34. PMID:
15471760.

12. Burns KE, Duffett M, Kho ME, Meade MO, Adhikari NK, Sinuff T, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ. 2008; 179(3):245–252. PMID:
18663204.

13. Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A consensus-based Checklist for Reporting of Survey Studies (CROSS). J Gen Intern Med. 2021; 36(10):3179–3187. PMID:
33886027.

14. Gaur PS, Zimba O, Agarwal V, Gupta L. Reporting survey based studies - a primer for authors. J Korean Med Sci. 2020; 35(45):e398. PMID:
33230988.

15. Maselli F, Esculier JF, Storari L, Mourad F, Rossettini G, Barbari V, et al. Low back pain among Italian runners: a cross-sectional survey. Phys Ther Sport. 2021; 48:136–145. PMID:
33434869.

16. Sousa GS, Fitzsimons MG, Mueller A, Quintão VC, Simões CM. Drug abuse amongst anesthetists in Brazil: a national survey. Braz J Anesthesiol. 2021; 71(4):326–332. PMID:
33845097.

17. Looijmans A, Spahrkäs SS, Sanderman R, Hagedoorn M. Ethical review procedures in international internet-based intervention studies. Internet Interv. 2021; 28:100487. PMID:
35646602.

18. Whicher D, Wu AW. Ethics review of survey research: a mandatory requirement for publication? Patient. 2015; 8(6):477–482. PMID:
26392006.

19. Langhaug LF, Sherr L, Cowan FM. How to improve the validity of sexual behaviour reporting: systematic review of questionnaire delivery modes in developing countries. Trop Med Int Health. 2010; 15(3):362–381. PMID:
20409291.

20. Artino AR Jr, Durning SJ, Sklar DP. Guidelines for reporting survey-based research submitted to academic medicine. Acad Med. 2018; 93(3):337–340. PMID:
29485492.

21. Teitcher JE, Bockting WO, Bauermeister JA, Hoefer CJ, Miner MH, Klitzman RL. Detecting, preventing, and responding to “fraudsters” in internet research: ethics and tradeoffs. J Law Med Ethics. 2015; 43(1):116–133. PMID:
25846043.

22. Palamar JJ, Acosta P. On the efficacy of online drug surveys during the time of COVID-19. Subst Abus. 2020; 41(3):283–285. PMID:
32697173.

23. Broadbent E, Wilkes C, Koschwanez H, Weinman J, Norton S, Petrie KJ. A systematic review and meta-analysis of the brief illness perception questionnaire. Psychol Health. 2015; 30(11):1361–1385. PMID:
26181764.

24. Vieira AM, Costa IZ, Oh P, Lima de Melo Ghisi G. Questionnaires designed to assess knowledge of heart failure patients: a systematic review. J Cardiovasc Nurs. 2016; 31(5):469–478. PMID:
26208265.

25. Cinar FI, Cinar M, Yilmaz S, Acikel C, Erdem H, Pay S, et al. Cross-cultural adaptation, reliability, and validity of the Turkish version of the compliance questionnaire on rheumatology in patients with Behçet’s disease. J Transcult Nurs. 2016; 27(5):480–486. PMID:
25801762.

26. Gao L, Zhang XC, Li MM, Yuan JQ, Cui XJ, Shi BX. Psychometric properties of the Chinese version of Arthritis Self-Efficacy Scale-8 (ASES-8) in a rheumatoid arthritis population. Rheumatol Int. 2017; 37(5):751–756. PMID:
28063069.

27. Park JI, Baek H, Kim SW, Jeong JY, Song KH, Yu JH, et al. Questionnaire-based survey of demographic and clinical characteristics, health behaviors, and mental health of young Korean adults with early-onset diabetes. J Korean Med Sci. 2021; 36(26):e182. PMID:
34227263.

28. Rodère M, Pereira B, Soubrier M, Fayet F, Piperno M, Pallot-Prades B, et al. Development and validation of a self-administered questionnaire measuring essential knowledge in patients with rheumatoid arthritis. Rheumatol Int. 2022; 42(10):1785–1795. PMID:
35389078.

29. Xiong X, Dalziel K, Huang L, Mulhern B, Carvalho N. How do common conditions impact health-related quality of life for children? Providing guidance for validating pediatric preference-based measures. Health Qual Life Outcomes. 2023; 21(1):8. PMID:
36698179.

30. Solomou I, Constantinidou F. Prevalence and predictors of anxiety and depression symptoms during the COVID-19 pandemic and compliance with precautionary measures: age and sex matter. Int J Environ Res Public Health. 2020; 17(14):4924. PMID:
32650522.

31. Fivecoat HC, Sayers SL, Riegel B. Social support predicts self-care confidence in patients with heart failure. Eur J Cardiovasc Nurs. 2018; 17(7):598–604. PMID:
29533083.

32. Zhao Y, Heida T, van Wegen EE, Bloem BR, van Wezel RJ. E-health support in people with Parkinson’s disease with smart glasses: a survey of user requirements and expectations in the Netherlands. J Parkinsons Dis. 2015; 5(2):369–378. PMID:
25855044.

33. DeSmet A, De Bourdeaudhuij I, Chastin S, Crombez G, Maddison R, Cardon G. Adults’ preferences for behavior change techniques and engagement features in a mobile app to promote 24-hour movement behaviors: cross-sectional survey study. JMIR Mhealth Uhealth. 2019; 7(12):e15707. PMID:
31859680.

34. Boon-Itt S, Skunkan Y. Public perception of the COVID-19 pandemic on twitter: sentiment analysis and topic modeling study. JMIR Public Health Surveill. 2020; 6(4):e21978. PMID:
33108310.

35. Ali SH, Foreman J, Capasso A, Jones AM, Tozan Y, DiClemente RJ. Social media as a recruitment platform for a nationwide online survey of COVID-19 knowledge, beliefs, and practices in the United States: methodology and feasibility analysis. BMC Med Res Methodol. 2020; 20(1):116. PMID:
32404050.

36. Stokes Y, Vandyk A, Squires J, Jacob JD, Gifford W. Using Facebook and LinkedIn to recruit nurses for an online survey. West J Nurs Res. 2019; 41(1):96–110. PMID:
29113542.

37. Lee JL, Choudhry NK, Wu AW, Matlin OS, Brennan TA, Shrank WH. Patient use of Email, Facebook, and physician websites to communicate with physicians: a national online survey of retail pharmacy users. J Gen Intern Med. 2016; 31(1):45–51. PMID:
26105675.

38. Wagner JP, Cochran AL, Jones C, Gusani NJ, Varghese TK Jr, Attai DJ. Professional use of social media among surgeons: results of a multi-institutional study. J Surg Educ. 2018; 75(3):804–810. PMID:
28964746.

39. Reich J, Guo L, Groshek J, Weinberg J, Chen W, Martin C, et al. Social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019; 25(3):587–591. PMID:
30203036.

40. Savard I, Kilpatrick K. Tailoring research recruitment strategies to survey harder-to-reach populations: a discussion paper. J Adv Nurs. 2022; 78(4):968–978. PMID:
35084799.

41. Hadler M, Klösch B, Reiter-Haas M, Lex E. Combining survey and social media data: respondents’ opinions on COVID-19 measures and their willingness to provide their social media account information. Front Sociol. 2022; 7:885784. PMID:
35874448.

42. Reich J, Guo L, Hall J, Tran A, Weinberg J, Groshek J, et al. A survey of social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016; 22(11):2678–2687. PMID:
27755269.

43. Bender JL, Hueniken K, Eng L, Brown MC, Kassirian S, Geist I, et al. Internet and social media use in cancer patients: association with distress and perceived benefits and limitations. Support Care Cancer. 2021; 29(9):5273–5281. PMID:
33651181.
44. Hlatshwako TG, Shah SJ, Kosana P, Adebayo E, Hendriks J, Larsson EC, et al. Online health survey research during COVID-19. Lancet Digit Health. 2021; 3(2):e76–e77. PMID:
33509387.
45. Ball HL. Conducting online surveys. J Hum Lact. 2019; 35(3):413–417. PMID:
31084575.
46. Smeds MR. A brief guide to survey methodology for vascular surgeons. Semin Vasc Surg. 2022; 35(4):431–437. PMID:
36414359.
47. McInroy LB. Pitfalls, potentials, and ethics of online survey research: LGBTQ and other marginalized and hard-to-access youths. Soc Work Res. 2016; 40(2):83–94. PMID:
27257362.

48. Stone DH. Design a questionnaire. BMJ. 1993; 307(6914):1264–1266. PMID:
8281062.

49. LaDonna KA, Taylor T, Lingard L. Why open-ended survey questions are unlikely to support rigorous qualitative insights. Acad Med. 2018; 93(3):347–349. PMID:
29215376.

50. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018; 93(3):360–366. PMID:
29210756.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download