INTRODUCTION
Acute hepatitis in children is caused by various infectious pathogens, including viruses. Most viral hepatitis cases improve spontaneously, except for a few, including hepatotropic viruses in children.
123 Therefore, pediatricians are often unaware of this self-resolving acute viral hepatitis.
4
The World Health Organization (WHO) reported 74 cases of acute hepatitis in children aged < 10 years, not caused by hepatitis A–E, between January 2022 and April 8, mostly in the United Kingdom (UK), during the coronavirus disease 2019 (COVID-19) pandemic, a unique inflection point for global health.
5 Since then, the WHO has reported that approximately 169 pediatric patients with acute hepatitis of unknown etiology have been diagnosed in 11 countries, including Spain, Israel, Denmark, Italy, the UK, and the United States of America (USA). In the reported cases, patients showed elevated aspartate transaminase (AST) or alanine transaminase (ALT) levels > 500 IU/L and jaundice without fever. Adenoviruses were detected in 74 cases, and a potential link with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) was considered.
6 A technical briefing from the UK Health Security Agency also reported 163 cases of acute non-A-E hepatitis in the UK, and 91 (72% of the tested cases) had adenoviruses. They hypothesized that the increased pediatric acute non-A-E hepatitis due to normal adenovirus infection with abnormal susceptibility or host response was because of a lack of exposure during the COVID-19 pandemic or a coinfection with SARS-CoV-2 or a toxin, drug, or environmental exposure.
7 In Alabama, USA, nine children with acute hepatitis without known causes were identified between October 1 and February 28, 2022. Among them, eight tested positive for adenovirus. Liver failure and liver transplantation (LT) rates were high (33.3% and 22.2%, respectively), and three patients with liver failure showed a higher adenoviral load.
8 Subsequently, next-generation sequencing revealed adenovirus-associated virus-2 (AAV2) in the plasma and liver of many patients with acute severe hepatitis of unknown etiology but not in healthy controls.
9 Previously, AAV2 was not considered a disease-causing virus but as a viral vector using in vitro or in vivo gene transfer. However, its discovery in many patients with non-A-E acute hepatitis in Europe and the USA has raised suspicions that it is a virus that can modify acute hepatitis. In addition, AAV2 may be associated with the prevalence of acute hepatitis as a helper virus of human adenovirus in some susceptible individuals having a human leukocyte antigen class II status.
9 Accordingly, an association between AAV2 and acute hepatitis of unknown etiology in children was suggested.
Therefore, monitoring the incidence of acute severe hepatitis of unknown etiology in Korea and providing appropriate diagnostic tests, including adenovirus and AAV2 polymerase chain reaction (PCR), are crucial. Accordingly, the Korea Disease Control and Prevention Agency (KDCA), Korean Society of Pediatrics, Korean Society of Pediatric Gastroenterology, Hepatology and Nutrition, and Korean Society of Pediatric Infectious Diseases established a nationwide surveillance system and conducted diagnostic tests to identify the cause of acute hepatitis having an infectious etiology. After this surveillance system was established, we performed this study to compare the incidence, severity, etiology, and prognosis of acute hepatitis of unknown etiology with those in the past in Korea. We systematically analyzed the results of our surveillance system regarding the status of acute severe hepatitis of unknown etiology in Korea.
METHODS
Study population and definition
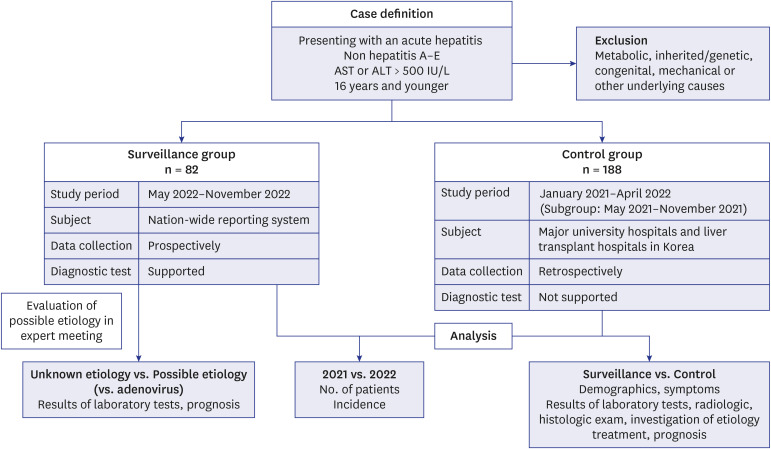
Acute severe hepatitis of unknown etiology was defined as a patient aged < 16 years with AST or ALT levels > 500 IU/L during the study period, not due to hepatitis A-E, and no metabolic, inherited/genetic, congenital, mechanical, or other underlying causes.
610 We collected data from two different groups: the surveillance and control groups (
Fig. 1). We established a nationwide surveillance system from May to November 2022. Using this surveillance system, we systematically collected prospective data and supporting diagnostic tests. The historical control group data were retrospectively collected from January 2021 to April 2022 for comparison with previous traditional acute hepatitis diagnoses and treatments. To reduce bias, such as seasonal variability and differences in study period, we analyzed patients who had visited the hospital from May to November 2021 as the control subgroup, which was the same month as that of the surveillance group. We included all LT centers and major university hospitals in all regions of South Korea as controls.
Fig. 1
A flow diagram of the current study of pediatric acute hepatitis of unknown etiology in Korea.
AST = aspartate transaminase, ALT = alanine transaminase.
Clinical and laboratory data
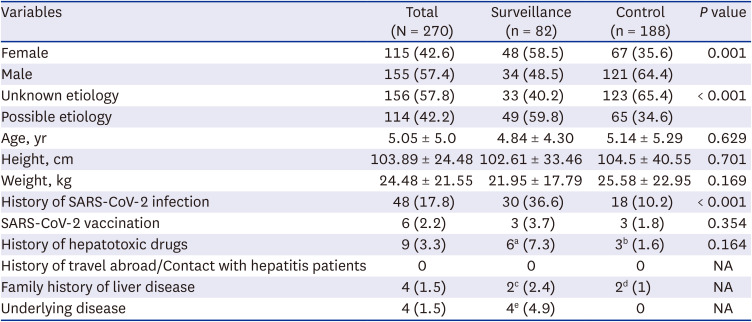
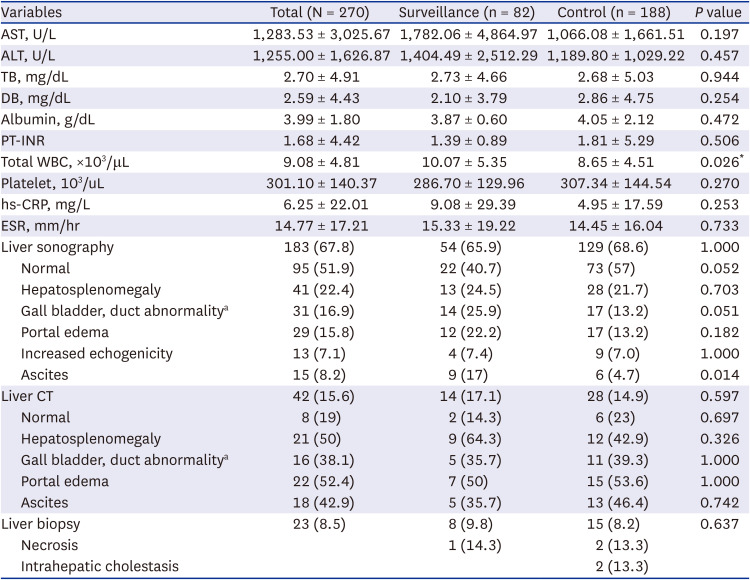
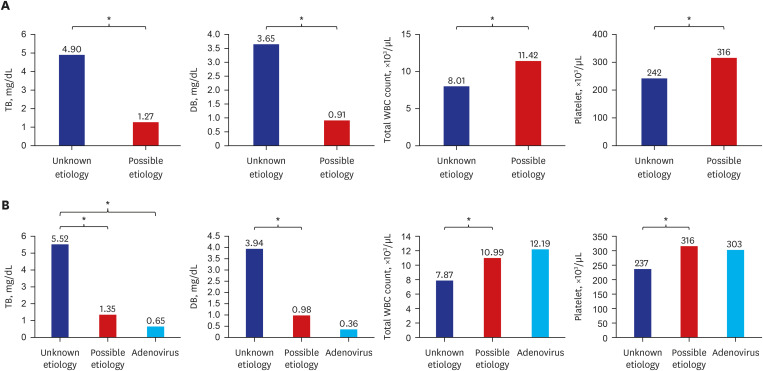
Demographic, biochemical, radiological, and histological data were collected from the surveillance and control groups. Major symptoms, history of SARS-CoV-2 infection and vaccination, underlying diseases, travel history, and family history of liver disease were recorded. Complete blood cell counts and liver function tests were used to collect biochemical data, including the maximum levels of AST, ALT, total bilirubin (TB), prothrombin time international normalized ratio (PT-INR), and albumin. Direct bilirubin (DB) was typically detected in patients with elevated TB levels. Liver ultrasonography and abdominal computed tomography (CT) findings were evaluated. Histological findings were evaluated in patients who underwent liver biopsy or LT. Hepatic necrosis, intranuclear inclusions, and adenovirus immunochemistry results were also evaluated.
11
We aimed to evaluate treatment modalities, severity, and prognosis of acute hepatitis of unknown etiology. Data on treatment modalities, such as antiviral agents, steroids, antibiotics, and intravenous immunoglobulins, were collected. The duration of admission to the general ward and the number of patients admitted to the intensive care unit (ICU) were recorded. Acute liver failure (ALF) was defined as coagulopathy not corrected with vitamin K and a PT-INR > 1.5 in patients with encephalopathy or as a PT-INR > 2 in patients with or without encephalopathy.
12 Data on the number of patients with ALF, LT, death, and recovery were collected.
Evaluation of possible etiologies
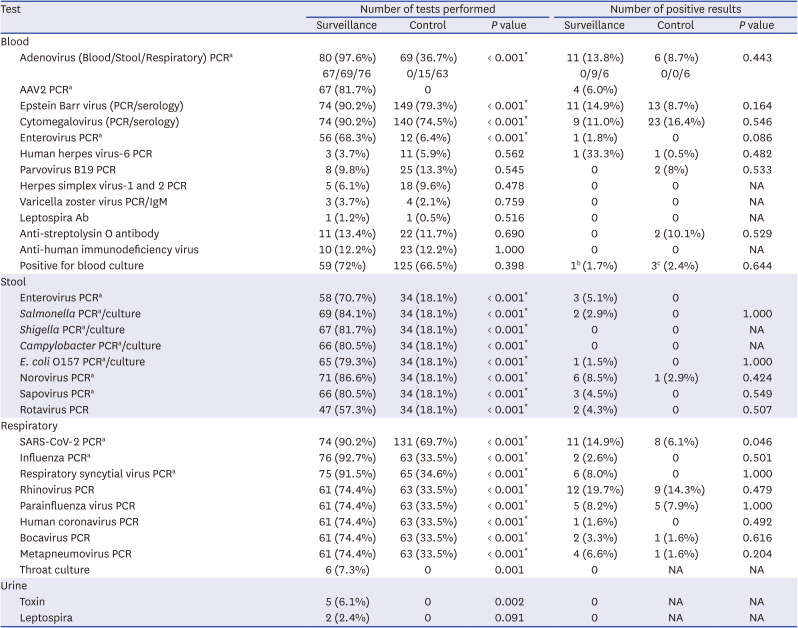
To investigate the possible etiology, we collected test results for possible pathogens, such as serological or molecular tests for hepatitis A-E, Epstein-Barr virus (EBV), cytomegalovirus (CMV), enterovirus, human herpes virus 6 (HHV-6), parvovirus B19, herpes simplex virus (HSV)-1, HSV-2, varicella-zoster virus,
leptospirosis, human immunodeficiency virus, and blood culture.
713 If respiratory specimens were available, the results of respiratory PCR for SARS-CoV-2, adenovirus, respiratory syncytial virus, influenza virus, rhinovirus, and parainfluenza virus were collected. The results of the stool culture or PCR were also collected, if available.
During surveillance, the KDCA tested whole blood, serum, respiratory, and stool samples for possible etiologies, including adenovirus, AAV2, and SARS-CoV-2. At the KDCA, all viral and bacterial tests were performed using PCR, and fecal bacterial tests were performed.
The AAV2 test was performed on whole blood samples obtained from the KDCA, and conventional PCR and gene sequencing were performed on positive samples after real-time PCR and next-generation sequencing (minimum detection limit of the gene detection method: 100 copies/mL).
An expert meeting was conducted monthly to evaluate the possible etiologies of hepatitis in the surveillance system. At the expert meeting, we checked the test results and clinical manifestations and classified the participants into subgroups by voting whether they had a possible etiology of hepatitis or not. Hepatotoxic drugs were evaluated as a possible etiology of hepatitis depending on the number and duration of medication use. Patients were classified as “unknown etiology” in absence of evidence of a possible hepatitis etiology, and as “possible etiology” if such evidence was present. We further classified our participants into three subgroups according to adenovirus detection: unknown etiology, possible etiology, and adenovirus.
Statistical analysis
The incidence was compared between 2021 and 2022 to confirm whether or not acute severe hepatitis of unknown etiology, which has been prevalent overseas since January 2022, was also present in Korea in 2022. However, other analyses were conducted for the surveillance and control groups because the study population, data collection methods, and supporting diagnostic tests differed between the two groups. The incidence was calculated by dividing the number of newly diagnosed acute hepatitis cases of unknown etiology by the number of Korean children aged 0–16 years in 2021 and 2022. Korean population data were extracted from the Korean Statistical Information Service (
https://kosis.kr/index/index.do).
Categorical variables, such as sex, etiology, symptoms, results of radiological and histological examinations, treatments, and severity, were analyzed using descriptive statistics, the chi-square test, and Fisher’s exact test. Continuous variables, such as age, admission duration, and laboratory test results, were compared using the analysis of variance with post hoc analysis or t-test. Non-parametric tests were used for non-normally distributed variables. Statistical analyses were performed using the SPSS Statistics software (version 27.0; IBM Corp., Armonk, NY, USA). Two-sided P < 0.05 were considered significant.
Ethics statement
The data were collected for disease surveillance by the KDCA, and because the data were reviewed retrospectively, the requirement for obtaining informed consent was waived. The study protocol was reviewed and approved by the institutional review boards of all included hospitals, including Pusan National University Yangsan Hospital (05-2023-045).
DISCUSSION
In this study, we established a nationwide surveillance system and performed diagnostic tests, including those for adenovirus and AAV2, for pediatric acute hepatitis of unknown etiology in Korea. We compared the incidence, severity, and prognosis of acute hepatitis of unknown etiology between surveillance and historical control groups.
Patients in the surveillance group were examined for infectious etiology more often than those in the control group, and significantly more possible etiologies were found. However, there were no significant differences between the two groups in terms of symptoms and signs; laboratory, radiological, and histological results; treatment; and prognosis. The surveillance group from May to November 2022 and the control group from January 2021 to April 2022 had similar pediatric acute hepatitis epidemiology. The surveillance and control groups showed no differences in possible etiologies.
Adenoviruses were detected in many pediatric patients with acute hepatitis of unknown etiology in Europe and the US from October 2021 to early 2022.
14 During the COVID-19 pandemic, since early 2020, there has been no adenovirus epidemic due to social distancing; however, a serious epidemic occurred when distancing measures were lifted in 2022. A cofactor in young children with adenoviral infections causes more severe infections or immune-mediated liver damage, which is usually mild.
15 However, a subsequent follow-up study concluded that the incidence of acute hepatitis of unknown etiology or LT in children in the USA will not increase significantly from 2017 to 2022.
16
In Korea, similar to Europe and the USA, the incidence of adenovirus infections has been suppressed since the onset of the COVID-19 pandemic. However, the incidence of adenovirus infections, especially enteric adenoviruses, has increased since early 2022.
17 In a 2018 Korean multicenter study of children with nonspecific reactive hepatitis, respiratory infection was the most common etiology, with 4.9% having adenovirus.
4 However, despite the increase in adenovirus infections, there was no difference in the frequency of adenovirus-related hepatitis between the surveillance (13.8%) and control (8.7%) groups in our study. In contrast, in the surveillance group, the unknown-etiology subgroup showed significantly higher TB and DB levels and significantly lower WBC and platelet counts than the adenovirus or possible-etiology subgroups, despite having similar prognosis. Adenovirus, which can cause hepatitis but is not clinically severe, is a major cause of hepatitis in children, although it is difficult to draw a conclusion due to the small number of patients. This finding indicates the need for continuous monitoring of adenoviruses in pediatric patients with acute hepatitis.
Pediatric acute severe hepatitis cases reported in Korea showed overall differences in clinical symptoms and prognoses compared with those reported in Europe and the USA. In terms of clinical symptoms, jaundice (69.2%) was the most frequent, followed by vomiting (57.9%), diarrhea (41.5%), and pale stools (40.5%) in cases reported in Europe,
18 whereas fever (23.1%) and respiratory symptoms (17.9%) were less frequent. In contrast, fever (68%) and respiratory symptoms, such as cough, sputum, and runny nose (17.0%), occurred frequently in Korean patients. In a European study,
19 of 208 patients with complete information regarding LT, 9.6% received or were waiting for LT, while 3.8% received LT, and most showed a favorable prognosis in our surveillance group. At the time of diagnosis, direct symptoms of hepatitis, such as jaundice, are the most commonly reported in Europe. In contrast, patients who visit the hospital because of fever or cold are often accidentally diagnosed with hepatitis after a laboratory examination in Korea likely owing to the relatively low medical cost. Therefore, the symptoms and prognosis differed even though patient information was collected using the same case definition.
AAV2 is a defective DNA virus that was not previously known to cause diseases. In the UK, AAV2 was tested using next-generation sequencing and real-time PCR, and AAV2 was detected in plasma and liver samples in 26 out of 32 (81%) cases of hepatitis compared with 5 out of 74 (7%) of samples from unaffected controls.
10 However, the effect of AAV2 in acute hepatitis remains unknown. In our study, 67 patients in the surveillance group were tested for AAV2 by plasma PCR, and AAV2 was confirmed in four. Three of the four patients with AAV2 were co-infected with another virus (adenovirus, enterovirus, or parainfluenza virus). One boy who underwent LT tested positive for AAV2; however, blood samples for AAV2 were obtained after LT. Therefore, it is uncertain whether AAV2 caused hepatitis. We could not test healthy controls for AAV2; therefore, we could not determine whether the AAV2 prevalence found in this study was significantly higher than that in the normal population.
We performed a subgroup analysis of the control group to eliminate bias, such as seasonal variability or differences in the study period between the two groups. Although we included the same month for the surveillance group and control subgroup, our main results were unchanged. Disease severity, presence of possible etiology, and prognosis did not differ significantly between 2021 and 2022, particularly from May to November. These results support our findings of no increase in the incidence of acute hepatitis of unknown etiology in 2022 compared to that in 2021 in Korea.
Our study had some limitations. If an infectious agent is detected, it may be difficult to determine whether it is the direct cause of hepatitis. In the group whose data were retrospectively investigated in 2021, tests to determine the etiology were insufficient. Although hepatitis E virus should have been excluded to meet these criteria, we did not exclude patients without hepatitis E results. However, the prevalence of hepatitis E in Korean children is extremely low (1% in 10–14-year-olds, 0% in 15–19-year-olds),
20 and it is unnecessary to assume that infection cannot be ruled out. Moreover, research on causes other than infection is lacking. Finally, the data for the control group were limited because they were collected retrospectively from the university hospitals where the researchers participated. However, there is a high probability that most of the omitted cases were mild, and the most severe cases were thought to have been reported, resulting in no significant difference in the conclusion.
This study had several strengths. To the best of our knowledge, this is the first nationwide report of acute hepatitis of unknown etiology in Asia using several diagnostic tests, including those for adenovirus, SARS-CoV-2, and AAV2. We found no outbreaks of severe acute hepatitis of unknown etiology associated with these viruses in Korea. We compared this study population among surveillance, control, and unknown and possible etiology subgroups and found that patients with an unknown etiology had higher disease severity and poorer prognosis.
In conclusion, this was the first Korean national study to investigate the incidence, causes, and prognosis of acute pediatric hepatitis. Severe acute hepatitis due to an adenovirus outbreak in the UK and USA was not observed in Korea. Pediatric acute hepatitis has a relatively fair prognosis; however, many cases of unknown etiology show high disease severity and poor prognosis.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download