This article has been
cited by other articles in ScienceCentral.
Abstract
Background
This retrospective observational matched-cohort study of 2,151,216 individuals from the Korean coronavirus disease 2019 (COVID-19) vaccine effectiveness cohort aimed to evaluate the comparative effectiveness of the COVID-19 bivalent versus monovalent vaccines in providing additional protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, critical infection, and death in Korea.
Methods
Among individuals, those vaccinated with COVID-19 bivalent vaccines were matched in a 1:1 ratio with those who were vaccinated with monovalent vaccines (bivalent vaccines non-recipients) during the observation period. We fitted a time-dependent Cox proportional-hazards model to estimate hazard ratios (HRs) of COVID-19 outcomes for infection, critical infection, and death, and we defined vaccine effectiveness (VE) as 1–HR.
Results
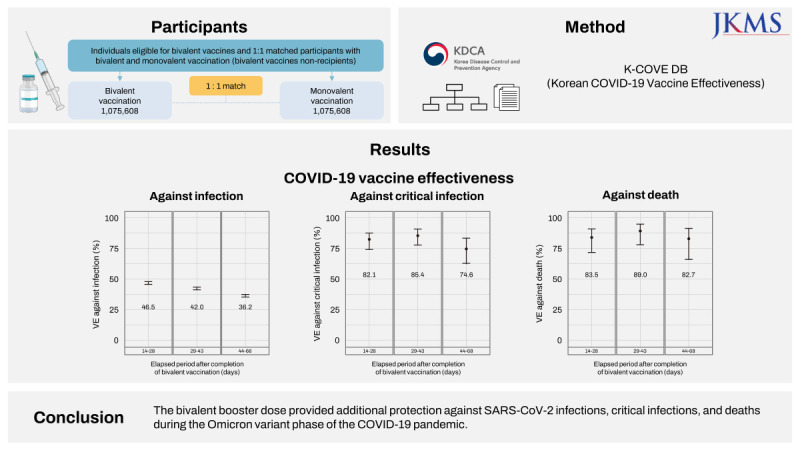
Compared with the bivalent vaccination group, the incidence proportions in the monovalent vaccination group were approximately three times higher for infection, nine times higher for critical infection, and 11 times higher for death. In the early stage of bivalent vaccination, relative VE of bivalent vaccine against monovalent vaccine was 42.4% against SARS-CoV-2 infection, 81.3% against critical infection, and 85.3% against death. In addition, VE against critical infection and death according to the elapsed period after bivalent vaccination was maintained at > 70%.
Conclusion
The bivalent booster dose provided additional protection against SARS-CoV-2 infections, critical infections, and deaths during the omicron variant phase of the COVID-19 pandemic.
Go to :

Graphical Abstract
Go to :

Keywords: Bivalent Vaccine, COVID-19, SARS-CoV-2, Vaccine Effectiveness
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron variant, first detected in late 2021, was highly contagious and many coronavirus disease 2019 (COVID-19)-confirmed cases have subsequently been reported.
123 The omicron variant was the dominant strain of SARS-CoV-2 in South Korea in early 2022.
4 The study about averted outcomes of COVID-19 for the monovalent vaccination showed that the national COVID-19 vaccination campaign in South Korea reduced the number of severe cases and deaths due to COVID-19.
5 With the surge in new variants, such as BQ.1.1, immunity derived from monovalent vaccines was inadequate to prevent infection owing to new variants; therefore, preparations had to be made for a COVID-19 resurgence in the winter of 2022.
6 The South Korean government implemented a winter vaccination program on October 11, 2022, and bivalent mRNA vaccination was primarily recommended, regardless of the type of vaccine previously administered.
Exceptions were made for individuals who were unsuitable vaccination candidates for mRNA-based vaccines, those who delayed being vaccinated with mRNA-based vaccines, and those who declined to be vaccinated with mRNA-based vaccines; they could receive recombinant protein-based vaccines such as NVX-CoV2373 and GBP510. When individuals were vaccinated with recombinant vaccines after they had completed the recommended vaccination according to age group, the last vaccination was recognized as the winter vaccination. Except for such cases, all vaccinations with the recombinant vaccine were considered third or fourth doses, rather than the winter vaccination. For improvement in the vaccination rate, the month starting on November 21, 2022 was targeted as an intensive vaccination period, and all efforts were made to vaccinate the South Korean population.
7
We conducted an observational study with the aim to evaluate the effectiveness of the COVID-19 bivalent vaccine compared to the monovalent vaccine in providing additional protection against SARS-CoV-2 infection, critical infection, and death in Korea.
Go to :

METHODS
Data source
In South Korea, all confirmed infections, severe cases, and deaths are reported to the government through the COVID-19 National Surveillance System, and COVID-19 vaccination records are reported and managed through the National Immunization Registry.
8
This study was based on data extracted from the Korean COVID-19 Vaccine Effectiveness (K-COVE) cohort. The K-COVE is an integrated data set comprising COVID-19 vaccine immunization registry data, national COVID-19 registry data, and severe disease and death data related to COVID-19, representing the country’s entire population. Detailed information on the K-COVE cohort has previously been reported.
9101112
Study design
This retrospective observational matched-cohort study aimed to evaluate the association between the bivalent booster vaccine and COVID-19 outcomes for infection, critical infection, and death by calculating vaccine effectiveness (VE) for the population vaccinated with the bivalent booster dose compared with those vaccinated with monovalent vaccines. The monovalent vaccination group means bivalent vaccines non-recipients during the observation period. The study commenced on October 25, 2022, which was 14 days after the bivalent vaccination campaign was implemented. mRNA-1273.214 was the primary supplied vaccine in South Korea during the study period.
We identified COVID-19-confirmed patients as those who tested positive for the SARS-CoV-2 virus based on the polymerase chain reaction, rapid antigen test, and emergency screening (emergency use-approved products), in accordance with the COVID-19 Response Guidelines in South Korea.
13 Patients with COVID-19-related severe disease and deaths were defined as previously described.
14 Critical infection was defined as severe disease or death due to COVID-19. Critical infections and deaths due to COVID-19 were limited to outcomes within 28 days of a positive COVID-19 test. We monitored patients included in this study until December 17, 2022 for infection, and until January 14, 2023 for critical infection and death.
Population
Inclusion criteria comprised individuals from the K-COVE cohort who were eligible for bivalent vaccines who were aged ≥ 18 years and who had completed at least the primary two-dose series of monovalent vaccination, and those who had received the most recent monovalent vaccine at least 90 days previously. We defined a completed vaccination as being 14 days post-vaccination and bivalent recipients as individuals who had received their bivalent vaccinations at least 14 days previously. Exclusion criteria comprised individuals who had confirmed COVID-19 prior to the start of the study period as well as those who had been reinfected with COVID-19. Among the bivalent vaccines, mRNA-1273.214 was the primary supplied vaccine during the study period. BNT162b2 Bivalent (WT/OMI BA.1) and BNT162b2 Bivalent (WT/OMI BA.4/BA.5) were introduced on November 7, 2022, and November 14, 2022, respectively. Therefore, the two BNT162b2 Bivalent vaccines were excluded from the analysis owing to an insufficient observation period.
Matched cohort study participants
Of the individuals eligible for bivalent boosters, those vaccinated with COVID-19 bivalent vaccines were matched in a 1:1 ratio with those who were vaccinated with monovalent vaccines (bivalent vaccines non-recipients) during the observation period. Individuals in each group were matched for sex, age group, place of residence, the number of previously administered monovalent doses, the period of time elapsed from the last dose of monovalent vaccines (3–4 months, 5–8 months, and ≥ 9 months), and a group based on individuals in long-term care facilities or immunocompromised conditions. We defined immunocompromised individuals as cancer patients, transplant patients, patients with primary immune deficiencies, patients with human immunodeficiency virus infections, and patients receiving high-dose corticosteroids or immunosuppressants.
Statistical analysis
Data pre-processing and all statistical analyses were conducted using R software (version 4.2.2; R Foundation, Vienna, Austria). We estimated the VE of COVID-19 bivalent vaccines according to the elapsed date of bivalent vaccination. We evaluated VE based on the number of monovalent vaccine doses previously received and the period of time elapsed from the last dose of the monovalent vaccine. We fitted a time-dependent Cox proportional-hazards model to estimate hazard ratios (HRs) of COVID-19 outcomes for infection, critical infection, and death, and defined VE as 1–HR.
Ethics statement
This study was conducted under the authority of the Korean Infectious Diseases Control and Prevention Act (Nos. 12,444 and 13,392) and the Institutional Review Board of Korea Disease Control and Prevention Agency approved the study (No. 2021-12-03-PE-A). The requirement of informed consent was waived owing to the study’s retrospective design.
Go to :

RESULTS
During the period between October 25 and December 17, 2022, among the 54,932,130 individuals in the K-COVE cohort, 18,088,019 met the eligibility criteria. Of these, 1,075,608 received a bivalent booster during the 54-day study period. Finally, 2,151,216 matched participants were included in the analysis (
Fig. 1).
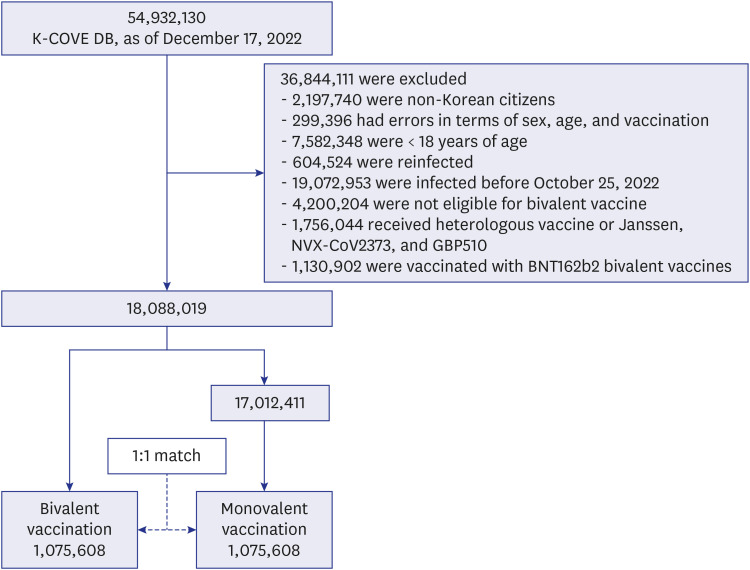 | Fig. 1
Definitions of the study population for the comparative effectiveness of the COVID-19 bivalent vaccine.
K-COVE = Korean COVID-19 Vaccine Effectiveness, COVID-19 = coronavirus disease 2019.

|
In
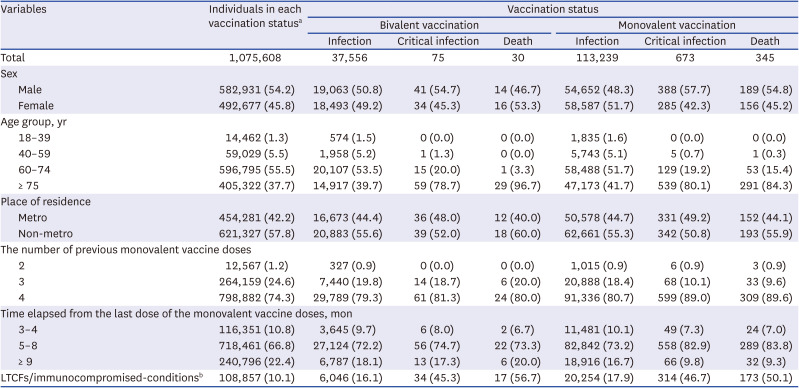
Table 1, the proportion of men vaccinated with bivalent vaccines was higher than that of women, and the proportion of participants in the metropolitan area was lower than that of participants in the non-metropolitan area. The proportion of those aged 60–74 years was the highest, at 55.5%, and the proportion of those who received their fourth dose of the monovalent vaccine was the highest, at 74.3%. The proportion of individuals for whom 5–8 months had elapsed after monovalent vaccination was the highest, at 66.8%. In the bivalent vaccination group, 37,556 infections (comprising 3.5% of the overall population), 75 critical infections, and 30 deaths were recorded. In the monovalent vaccination group, 113,239 infections (10.5% of the overall population), 673 critical infections, and 345 deaths were reported. In each group, critical infections and deaths comprised a small percentage of cases (< 0.1%). Compared with the bivalent vaccination group, the simple incidence proportions in the monovalent vaccination group were approximately three times higher for infection, nine times higher for critical infection, and 11 times higher for death.
Table 1
Baseline characteristics of matched participants included in the analysis (N = 2,151,216)
|
Variables |
Individuals in each vaccination statusa
|
Vaccination status |
|
Bivalent vaccination |
Monovalent vaccination |
|
Infection |
Critical infection |
Death |
Infection |
Critical infection |
Death |
|
Total |
1,075,608 |
37,556 |
75 |
30 |
113,239 |
673 |
345 |
|
Sex |
|
|
|
|
|
|
|
|
Male |
582,931 (54.2) |
19,063 (50.8) |
41 (54.7) |
14 (46.7) |
54,652 (48.3) |
388 (57.7) |
189 (54.8) |
|
Female |
492,677 (45.8) |
18,493 (49.2) |
34 (45.3) |
16 (53.3) |
58,587 (51.7) |
285 (42.3) |
156 (45.2) |
|
Age group, yr |
|
|
|
|
|
|
|
|
18–39 |
14,462 (1.3) |
574 (1.5) |
0 (0.0) |
0 (0.0) |
1,835 (1.6) |
0 (0.0) |
0 (0.0) |
|
40–59 |
59,029 (5.5) |
1,958 (5.2) |
1 (1.3) |
0 (0.0) |
5,743 (5.1) |
5 (0.7) |
1 (0.3) |
|
60–74 |
596,795 (55.5) |
20,107 (53.5) |
15 (20.0) |
1 (3.3) |
58,488 (51.7) |
129 (19.2) |
53 (15.4) |
|
≥ 75 |
405,322 (37.7) |
14,917 (39.7) |
59 (78.7) |
29 (96.7) |
47,173 (41.7) |
539 (80.1) |
291 (84.3) |
|
Place of residence |
|
|
|
|
|
|
|
|
Metro |
454,281 (42.2) |
16,673 (44.4) |
36 (48.0) |
12 (40.0) |
50,578 (44.7) |
331 (49.2) |
152 (44.1) |
|
Non-metro |
621,327 (57.8) |
20,883 (55.6) |
39 (52.0) |
18 (60.0) |
62,661 (55.3) |
342 (50.8) |
193 (55.9) |
|
The number of previous monovalent vaccine doses |
|
|
|
|
|
|
|
|
2 |
12,567 (1.2) |
327 (0.9) |
0 (0.0) |
0 (0.0) |
1,015 (0.9) |
6 (0.9) |
3 (0.9) |
|
3 |
264,159 (24.6) |
7,440 (19.8) |
14 (18.7) |
6 (20.0) |
20,888 (18.4) |
68 (10.1) |
33 (9.6) |
|
4 |
798,882 (74.3) |
29,789 (79.3) |
61 (81.3) |
24 (80.0) |
91,336 (80.7) |
599 (89.0) |
309 (89.6) |
|
Time elapsed from the last dose of the monovalent vaccine doses, mon |
|
|
|
|
|
|
|
|
3–4 |
116,351 (10.8) |
3,645 (9.7) |
6 (8.0) |
2 (6.7) |
11,481 (10.1) |
49 (7.3) |
24 (7.0) |
|
5–8 |
718,461 (66.8) |
27,124 (72.2) |
56 (74.7) |
22 (73.3) |
82,842 (73.2) |
558 (82.9) |
289 (83.8) |
|
≥ 9 |
240,796 (22.4) |
6,787 (18.1) |
13 (17.3) |
6 (20.0) |
18,916 (16.7) |
66 (9.8) |
32 (9.3) |
|
LTCFs/immunocompromised-conditionsb
|
108,857 (10.1) |
6,046 (16.1) |
34 (45.3) |
17 (56.7) |
20,254 (17.9) |
314 (46.7) |
173 (50.1) |

A comparison between COVID-19 bivalent and monovalent vaccines
The VE of the bivalent vaccination was 42.4% against infection (95% confidence interval [CI], 41.7–43.1) from October 25, 2022 to December 17, 2022. The VE of the bivalent vaccination was 81.3% against critical infection (95% CI, 76.3–85.3); and 85.3% against death (95% CI, 78.5–89.9) from October 25, 2022 to January 14, 2023. We examined time-varying VE by 14–28, 29–43, and 44–68 days after the bivalent vaccination to determine the waning of protection. Each time-varying VE of bivalent vaccination compared with monovalent vaccination was calculated, and each
P value was < 0.001. VE against infection decreased over time, 46.5% (95% CI, 45.5–47.4), 42.0% (95% CI, 40.9–43.0), and 36.2% (95% CI, 34.9–37.5), whereas VE against critical infection and death were maintained at 74.6% (95% CI, 62.2–82.9) and 82.7% (95% CI, 65.9–91.2), respectively (
Fig. 2).
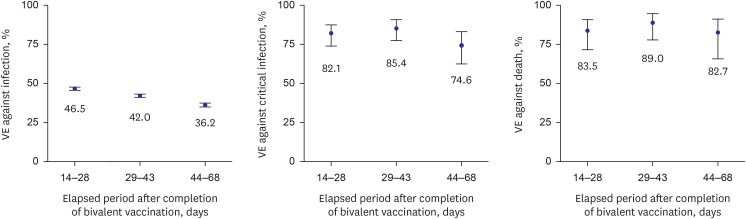 | Fig. 2
Time-varying COVID-19 VE against infection, critical infection, and death in individuals who received bivalent vaccine compared with those who received monovalent vaccine. Error bars indicate 95% CIs.
VE = vaccine effectiveness, COVID-19 = coronavirus disease 2019, CI = confidence interval.

|
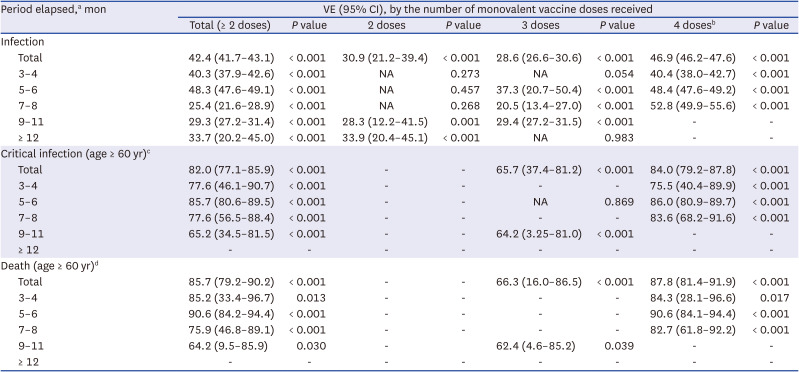
The start date of the fourth vaccination was February 14, 2022; therefore, a time period longer than 9 months had not elapsed following the fourth dose for any participant. We further examined the risk of COVID-19 outcomes in subgroups of cohorts stratified by the period elapsed from the most recent monovalent vaccine dose and the number of monovalent vaccine doses. Only approximately five critical infections and deaths were observed in those aged < 60 years; therefore, we estimated VE in relation to critical infections and deaths for individuals aged ≥ 60 years.
Table 2 shows that no critical infection or death occurred after bivalent vaccination and two-dose vaccination; therefore, VE against critical infection and death in these subgroups was not estimated. The second and third vaccinations have been conducted from 2021, and few new vaccinations have been administered in the 3–8 months prior to the end of the study period (< 1,200 individuals); therefore, the VE for specific subgroups was not applicable. A longer elapsed period after monovalent vaccination was associated with a higher VE against infection in all monovalent vaccine statuses, ranging from 40.4% to 52.8%, in the four vaccine doses. In terms of critical infections and deaths among participants aged ≥ 60 years, VE was maintained at > 60% in all cases. In particular, in four doses, the VE for critical infection was maintained between 75.5% and 86.0%, and VE against death was maintained between 82.7% and 90.6%.
Table 2
Subgroup analysis for bivalent vaccination versus monovalent vaccination by period elapsed and the number of monovalent vaccine doses
|
Period elapsed,a mon |
VE (95% CI), by the number of monovalent vaccine doses received |
|
Total (≥ 2 doses) |
P value |
2 doses |
P value |
3 doses |
P value |
4 dosesb
|
P value |
|
Infection |
|
|
|
|
|
|
|
|
|
Total |
42.4 (41.7–43.1) |
< 0.001 |
30.9 (21.2–39.4) |
< 0.001 |
28.6 (26.6–30.6) |
< 0.001 |
46.9 (46.2–47.6) |
< 0.001 |
|
3–4 |
40.3 (37.9–42.6) |
< 0.001 |
NA |
0.273 |
NA |
0.054 |
40.4 (38.0–42.7) |
< 0.001 |
|
5–6 |
48.3 (47.6–49.1) |
< 0.001 |
NA |
0.457 |
37.3 (20.7–50.4) |
< 0.001 |
48.4 (47.6–49.2) |
< 0.001 |
|
7–8 |
25.4 (21.6–28.9) |
< 0.001 |
NA |
0.268 |
20.5 (13.4–27.0) |
< 0.001 |
52.8 (49.9–55.6) |
< 0.001 |
|
9–11 |
29.3 (27.2–31.4) |
< 0.001 |
28.3 (12.2–41.5) |
0.001 |
29.4 (27.2–31.5) |
< 0.001 |
- |
- |
|
≥ 12 |
33.7 (20.2–45.0) |
< 0.001 |
33.9 (20.4–45.1) |
< 0.001 |
NA |
0.983 |
- |
- |
|
Critical infection (age ≥ 60 yr)c
|
|
|
|
|
|
|
|
|
|
Total |
82.0 (77.1–85.9) |
< 0.001 |
- |
- |
65.7 (37.4–81.2) |
< 0.001 |
84.0 (79.2–87.8) |
< 0.001 |
|
3–4 |
77.6 (46.1–90.7) |
< 0.001 |
- |
- |
- |
- |
75.5 (40.4–89.9) |
< 0.001 |
|
5–6 |
85.7 (80.6–89.5) |
< 0.001 |
- |
- |
NA |
0.869 |
86.0 (80.9–89.7) |
< 0.001 |
|
7–8 |
77.6 (56.5–88.4) |
< 0.001 |
- |
- |
- |
- |
83.6 (68.2–91.6) |
< 0.001 |
|
9–11 |
65.2 (34.5–81.5) |
< 0.001 |
- |
- |
64.2 (3.25–81.0) |
< 0.001 |
- |
- |
|
≥ 12 |
- |
- |
- |
- |
- |
- |
- |
- |
|
Death (age ≥ 60 yr)d
|
|
|
|
|
|
|
|
|
|
Total |
85.7 (79.2–90.2) |
< 0.001 |
- |
- |
66.3 (16.0–86.5) |
< 0.001 |
87.8 (81.4–91.9) |
< 0.001 |
|
3–4 |
85.2 (33.4–96.7) |
0.013 |
- |
- |
- |
- |
84.3 (28.1–96.6) |
0.017 |
|
5–6 |
90.6 (84.2–94.4) |
< 0.001 |
- |
- |
- |
- |
90.6 (84.1–94.4) |
< 0.001 |
|
7–8 |
75.9 (46.8–89.1) |
< 0.001 |
- |
- |
- |
- |
82.7 (61.8–92.2) |
< 0.001 |
|
9–11 |
64.2 (9.5–85.9) |
0.030 |
- |
|
62.4 (4.6–85.2) |
0.039 |
- |
- |
|
≥ 12 |
- |
- |
- |
- |
- |
- |
- |
- |

Go to :

DISCUSSION
In this retrospective, observational, matched-cohort study, we estimated the effectiveness of the COVID-19 bivalent mRNA vaccine compared to the monovalent mRNA vaccine against SARS-CoV-2 infection, critical infection, and death in South Koreans aged ≥ 18 years who were eligible for the COVID-19 bivalent vaccine. This study identified a lower risk of SARS-CoV-2 infection, critical infection, and death after receipt of the bivalent vaccine during the time of omicron variant circulation among South Koreans.
VE against infection was reduced from 46.5% to 36.2%, whereas VE against critical infection and death was maintained above approximately 75% and 83%, respectively, which is similar to previous study findings.
15 We found that with a greater number of vaccine doses and an increased elapsed time period after the most recent monovalent vaccine dose, the additional protective effectiveness of bivalent vaccination against monovalent vaccination tended to increase, which is similar to previously reported findings in a United States study.
16
The specific subgroups among individuals vaccinated with the bivalent vaccine after two and three doses of monovalent vaccinations showed no significant associations between VE and the elapsed time period after monovalent vaccinations, most likely because the number of individuals in each group was relatively small. However, a longer elapsed period after the fourth vaccination with the monovalent vaccine was associated with a higher VE of bivalent vaccination (40.4–52.8%).
We also observed that, after four doses, VE at 7–8 months was lower than VE at 5–6 months for critical infection and death. This result could be because the immunocompromised and individuals in long-term care facilities were the first to be vaccinated with the fourth dose.
To our knowledge, this study is the first in South Korea to evaluate the relative VE of the bivalent vaccine against monovalent vaccines in relation to vaccination and COVID-19 outcomes across the entire population. However, this study has some limitations. Individuals with hybrid immunity had the highest magnitude of protection.
17 As infections prior to the start date of the analysis were excluded, a combination of vaccination and infection (hybrid immunity) could not be considered because the previous infection history was not reflected. Underlying medical conditions were found to be risk factors for severe COVID-19 infection and death, but our study findings did not reflect them.
18
The use of BNT162b2 Bivalent (WT/OMI BA.1) for vaccination began on November 7, 2022, and the use of BNT162b2 Bivalent (WT/OMI BA.4/BA.5) began on November 14, 2022. The observation period in relation to the two BNT162b2 Bivalent vaccines was insufficient; therefore, those who were vaccinated with a bivalent vaccine other than mRNA-1273.214 were excluded from the analysis. Therefore, generalizing our findings to other bivalent vaccines should be undertaken with caution. Future studies with extended observation periods covering the entire winter season and an evaluation of other bivalent vaccine types should be conducted.
In this study, the bivalent booster dose provided additional protection against SARS-CoV-2 infections, critical infections, and deaths during the omicron variant phase of the COVID-19 pandemic. Therefore, bivalent vaccination should be consistently recommended, especially to individuals aged ≥ 60 years and individuals who have not been vaccinated for a long time. Further consideration of the effects of bivalent vaccination is needed as this study only included those vaccinated with mRNA-1273.214. In future studies, we intend to conduct an analysis by expanding the observation period and including other types of bivalent vaccines.
Go to :

ACKNOWLEDGMENTS
We thank the COVID-19 Vaccination Task Force and Division of National Immunization, Korea Disease Control and Prevention Agency; relevant ministries, including the Ministry of Interior and Safety, Si/Do and Si/Gun/Gu; medical staff in health centers; and medical facilities for their efforts in responding to the COVID-19 outbreak. This study is a part of the Korea COVID-19 Vaccine Effectiveness (K-COVE) study, which was initiated and operated by the Korea Disease Control and Prevention Agency.
Go to :

Notes
Go to :

References
1. Onishchenko GG, Sizikova TE, Lebedev VN, Borisevich SV. The Omicron variant of the SARS-CoV-2 virus as the dominant agent of a new risk of disease amid the COVID-19 pandemic. Herald Russ Acad Sci. 2022; 92(4):381–391.

3. Chalkias S, Feng J, Chen X, Zhou H, Marshall JC, Girard B, et al. Neutralization of omicron subvariant BA.2.75 after bivalent vaccination. N Engl J Med. 2022; 387(23):2194–2196. PMID:
36416761.

4. Lim S, Sohn M. How to cope with emerging viral diseases: lessons from South Korea’s strategy for COVID-19, and collateral damage to cardiometabolic health. Lancet Reg Health West Pac. 2023; 30:100581. PMID:
36093123.

5. Hwang JH, Lee JH, Jang EJ, Kim RK, Lee KH, Park SK, et al. Estimating the number of severe COVID-19 cases and COVID-19-related deaths averted by a nationwide vaccination campaign in Republic of Korea. Osong Public Health Res Perspect. 2023; 14(3):164–172. PMID:
37415433.

6. Uraki R, Ito M, Furusawa Y, Yamayoshi S, Iwatsuki-Horimoto K, Adachi E, et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect Dis. 2023; 23(1):30–32. PMID:
36495917.

8. Yoo KJ, Kwon S, Choi Y, Bishai DM. Systematic assessment of South Korea’s capabilities to control COVID-19. Health Policy. 2021; 125(5):568–576. PMID:
33692005.
9. Jang EJ, Choe YJ, Kim RK, Park YJ. BNT162b2 Vaccine effectiveness against the SARS-CoV-2 Omicron variant in children aged 5 to 11 years. JAMA Pediatr. 2023; 177(3):319–320. PMID:
36622683.
10. Kim YY, Choe YJ, Kim J, Kim RK, Jang EJ, Lee H, et al. Vaccine effectiveness against severe disease and death for patients with COVID-19 during the delta-dominant and omicron-emerging periods: a K-COVE study. J Korean Med Sci. 2023; 38(11):e87. PMID:
36942395.

11. Kim J, Choe YJ, Jang EJ, Lim DS, Kim YY, Kim RK, et al. Effectiveness of booster mRNA vaccines against SARS-CoV-2 infection in an elderly population, South Korea, October 2021–January 2022. Clin Infect Dis. 2022; 75(5):920–921. PMID:
35439294.

12. Kim YY, Choe YJ, Kim J, Kim RK, Jang EJ, Park SK, et al. Effectiveness of second mRNA COVID-19 booster vaccine in immunocompromised persons and long-term care facility residents. Emerg Infect Dis. 2022; 28(11):2165–2170. PMID:
36191615.

13. Korea Disease Control and Prevention Agency. COVID-19 vaccination. Updated 2023. Accessed May 9, 2023.
https://ncv.kdca.go.kr/eng/
.
14. Yi S, Choe YJ, Lim DS, Lee HR, Kim J, Kim YY, et al. Impact of national COVID-19 vaccination campaign, South Korea. Vaccine. 2022; 40(26):3670–3675. PMID:
35570077.

15. Arbel R, Peretz A, Sergienko R, Friger M, Beckenstein T, Yaron S, et al. Effectiveness of the bivalent mRNA vaccine in preventing severe COVID-19 outcomes: an observational cohort study. Lancet. 2023.

16. Link-Gelles R, Ciesla AA, Fleming-Dutra KE, Smith ZR, Britton A, Wiegand RE, et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection— increasing community access to testing program, United States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(48):1526–1530. PMID:
36454688.

17. Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023; 23(5):556–567. PMID:
36681084.

18. Shi HJ, Yang J, Eom JS, Ko JH, Peck KR, Kim UJ, et al. Clinical characteristics and risk factors for mortality in critical COVID-19 patients aged 50 years or younger during Omicron wave in Korea: comparison with patients older than 50 years of age. J Korean Med Sci. 2023; 38(28):e217. PMID:
37463688.

Go to :







 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download