Abstract
The current emergence of the coronavirus disease 2019 (COVID-19) pandemic and the possible side effects of COVID-19 mRNA vaccination remain worrisome. Few cases of vaccination-related side effects, such as vasculitis, have been reported. Eosinophilic granulomatosis with polyangiitis (EGPA), also known as Churg-Strauss syndrome, is a type of vasculitis characterized by the histological richness of eosinophils, asthma, polyneuropathy, sinusitis, and skin or lung involvement. Here, we report the first case of new onset EGPA following COVID-19 vaccination in Korea. A 71-year old woman developed a skin rash and presented with progressive weakness of the upper and lower extremities after the BNT162b2 vaccination (Pfizer-BioNTech). She was diagnosed with EGPA and her symptoms improved after systemic steroid and immunosuppressant therapy. Although it is very rare, clinicians should be aware that EGPA may occur after COVID-19 vaccination.
Go to : 
Graphical Abstract
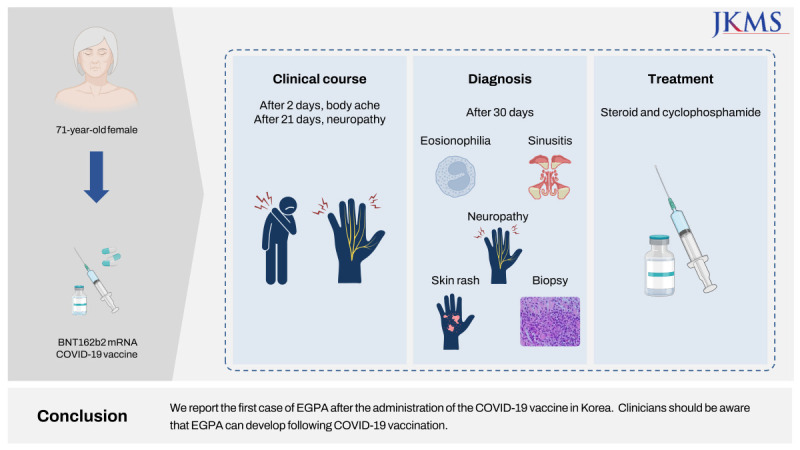
Go to : 
The coronavirus disease 2019 (COVID-19) pandemic had really affected the entire human life in the world. A new type of vaccine such as mRNA vaccines was also firstly introduced. Although the COVID-19 pandemic is over, there is still a need for vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The anti-SARS-CoV-2 mRNA vaccines are safe and effective; however, rare cases of serious adverse effects have been reported, including vasculitis and immune-mediated diseases (IMDs).12 The exact mechanisms of vaccine-induced IMD progression are unknown, and it is still debated whether it is a causal association or a mere coincidence. During the COVID-19 pandemic, some forms of vasculitis were observed during or after COVID-19 infection and recovery, and several cases of vaccine-related anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) were reported following vaccination or a booster dose.345 Recently, it has been reported that eosinophilic granulomatosis with polyangiitis (EGPA) was diagnosed after the second dose of COVID-19 vaccination, and early diagnosis on patients with EGPA had a recurrence of severe polyneuropathy after vaccination.67 Here, we report the first case of de novo EGPA after COVID-19 vaccination in Korea.
Go to : 
A 71-year-old woman with a history of diabetes mellitus type 2, visited the emergency department because of right shoulder pain and motor weakness with a generalized rash.
The patient had experienced intermittent cough for 2 years (since December 2020). She occasionally experienced wheezing when emotionally aroused. After receiving oral medication for cough, she developed a skin rash with itching. However, because the symptoms waxed and waned, she did not receive any treatment for the symptoms.
She received the first dose of BNT162b2 mRNA COVID-19 vaccination (Pfizer-BioNTech) on October 8, 2021, at a local center. Approximately 2 days after vaccination, she developed a severe body ache and took medication for a few days for muscle pain, including non-steroidal anti-inflammatory drugs. Three weeks later, both fingers and the entire sole were tingling, and the feeling of a balloon inflating under the sole was accompanied by numbness. At the same time, neither ankle was lifted and there was no sensation below the ankles.
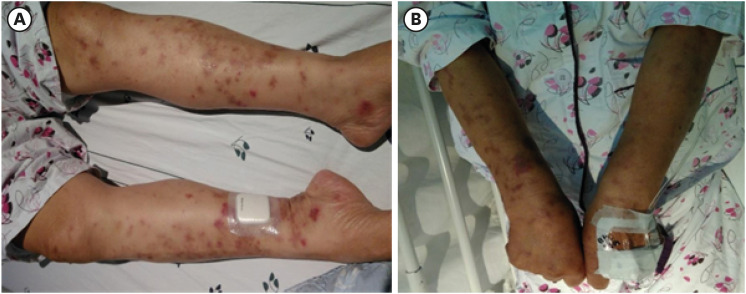
One week after the event (one month after vaccination), she took a blood test which revealed peripheral blood eosinophilia (white blood cell count 25,000/uL, eosinophil 52.9%) at a nearby local clinic. She was referred the emergency room because of tearing pain in her right shoulder and weakness in her right upper arm with eosinophilia. Upon visiting the hospital, the patient experienced weakness in the lower extremities. She also presented with erythematous patch lesions on both lower arms and legs (Fig. 1). She also complained of pain and numbness in both palms and feet, which were associated with poor balance. The patient’s symptoms worsened progressively.
Neurological examination revealed an asymmetric upper motor neuron pattern of weakness affecting the upper limbs with right shoulder and elbow. She also showed signs of foot drop without loss of deep tendon reflexes in the lower limbs. The patient did not exhibit truncal ataxia. Romberg test results were negative, with no other cerebellar signs. No pupillary defects are observed.
Laboratory tests revealed eosinophilia (43%, absolute count 10,350/uL) and increased white blood cell count 24,080/uL, and erythrocyte sedimentation rate 44/h). Anti-myeloperoxidase (perinuclear-ANCA [p-ANCA]) level was > 135 RU/m and antinuclear antibodies was negative. Tests for anti-CCP, rheumatoid factor, and antiphospholipid antibodies were not conducted. Renal and liver function test results were unremarkable. Magnetic resonance imaging findings of the brain were unremarkable. A nerve conduction study revealed right median, ulnar, peroneal, and posterior tibial neuropathy with absent sural conduction. A nerve motor-evoked potential study also showed a peripheral conduction defect in both posterior tibial nerves.
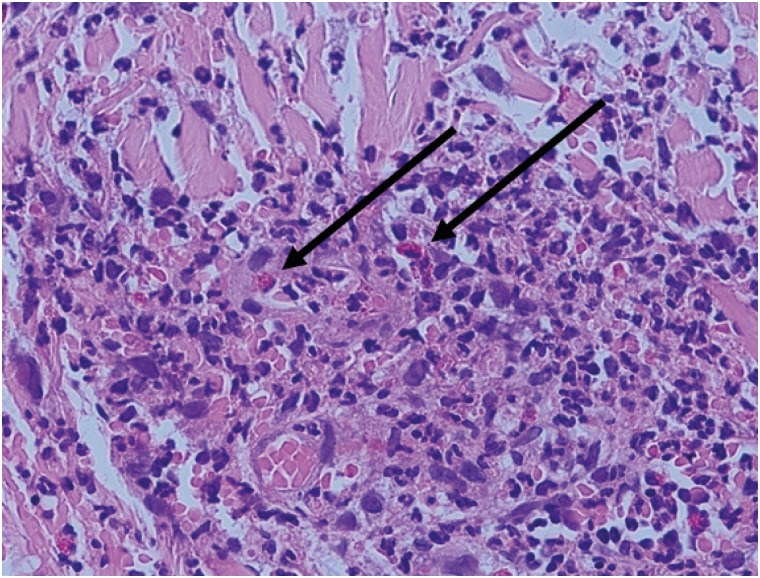
Skin biopsy of the calf showed perivascular neutrophilic, eosinophilic, and some lymphocytic infiltration (Fig. 2). Chest radiography findings were unremarkable. Radiographs of the paranasal sinuses revealed bilateral maxillary sinusitis.
She was diagnosed with new-onset EGPA or Churg-Strauss syndrome (based on the diagnostic criteria of the American College of Rheumatology [ACR]/European Alliance of Associations for Rheumatology Classification [EULAR], with eosinophilia, neuropathy, paranasal sinus abnormality, and histological findings).89
The patient was treated with intravenous methylprednisolone 1 mg/kg (60 mg) three times a day for 3 days and cyclophosphamide 75 mg orally. After the initiation of systemic corticosteroid therapy, we observed a dramatic decrease of blood eosinophils (from eosinophil 43% to 3.3% [absolute eosinophil count 10,350/uL to 430/uL] after first steroid therapy). The numbness of both feet, foot drop sign of the right limb, and rash were also improved. The steroid dose was reduced and maintained at 120 mg (60 mg twice a day) for 3 days and then 60 mg daily for 1 week. Then oral steroid dose was tapered 5 mg every 1–2 weeks depending on clinical progress.
After 62 days of systemic steroid treatment following discharge, cyclophosphamide was discontinued because of the improvement of symptoms and leukopenia (blood cell count was decreased from white blood cell count 9,240/uL to 370/uL in 2 weeks). Finally, her motor power almost recovered and was maintained with oral prednisolone (10 mg every other day) 200 days after discharge.
Ethics committee approval was received for this study from the Institutional Review Board (IRB) of the Seoul National University of Bundang on 13 May 2023 (IRB No. B-2305-829-701). Informed consent for data collection and publication of the study was obtained from the case patient.
Go to : 
The COVID-19 vaccine is generally considered safe and effective in the general population. The adverse reactions to COVID-19 vaccines are usually mild and include injection site pain, myalgia, fever, chills, and headache. However, it has been reported that severe symptoms could be involved in internal organs and be life-threatening in rare cases like vasculitis, Guillain-Barre syndrome, and myocarditis.10
EGPA, formerly known as “Churg-Strauss syndrome,” is a small vessel AAVs, which is a multi-organ disorder.11 The prevalence in the general population is 10.7–13 cases per million populations. The annual incidence of EGPA is 0.5–6.8 new cases/year per million populations. It mostly affects subjects between 40 and 60 years of age, and the mean age at diagnosis is 48 years, without a clear sex predominance.12
EGPA is classically described to evolve into three phases. The first phase is a prodromal phase characterized by respiratory allergies, such as asthma and rhinosinusitis, and the second phase is an eosinophilic phase characterized by peripheral eosinophilia and organ involvement. Third vasculitis phase with clinical manifestations of small-vessel vasculitis.11
The diagnosis of EGPA mainly relies on clinical manifestations. Specific criteria are available for the diagnosis of EGPA. This established tool is helpful for classifying EGPA in vasculitis. The classification criteria include asthma, eosinophilia, neuropathy, pulmonary infiltrates that are not fixed, paranasal sinus abnormalities and extravascular eosinophils, and four out of six symptoms should be present.8 The recently revised diagnostic criteria for EGPA include seven clinical features: clinically obstructive airway disease, nasal polyps, mononeuritis multiplex, blood eosinophilia and evidence of extravascular eosinophilic inflammation, without the presence of cytoplasmic-ANCA or hematuria. A score of 6 or higher is considered indicative of EGPA.9
Commonly, most patients present with asthma, sinusitis, allergic rhinitis, or nasal polyposis. The most frequently involved site in asthma is the respiratory system, and pathological confirmation of eosinophil infiltration in the lungs is useful for histological diagnosis. During the vasculitic phase, skin manifestations such as subcutaneous nodules and purpura, especially involving the legs, are clinical features. Skin biopsies generally show leukocytoclastic vasculitis.11
Peripheral neuropathy, such as mononeuritis multiplex, is associated with axonal injury, which is usually unilateral and asymmetric, and is the most characteristic manifestation of peripheral nervous system involvement. During the vasculitis phase, areas of the peroneal, tibial, and ulnar nerves are affected and patients report paresthesia and pain.11
The patient had chronic cough with intermittent wheezing sound, which suggested that she might have asthma. However, an evaluation of asthma could not be conducted because treatment was urgently needed. The patient presented with polyneuropathy with general weakness, peripheral eosinophilia, and maxillary sinusitis. A skin biopsy of a blood vessel showed an accumulation of eosinophils.
EGPA needs to be differentially diagnosed from other vascular and eosinophilic disorders. Other small-vessel AAVs, such as granulomatosis with polyangiitis and microscopic polyangiitis, may have similar clinical features. However, they are not characterized by an underlying condition of asthma or increased eosinophils in the peripheral blood or tissues.13 Hypereosinophilic syndrome (HES) may share some features such as hypereosinophilia in the peripheral blood and eosinophilic involvement of organs or tissues. If the absolute eosinophil count is greater than 1,500/uL, systemic symptoms are present, but no evidence of vasculitis is found on tissue or organ biopsy, it is most likely a case of HES.14
In this case, the patient met both the classic ACR/EULAR classification criteria for EGPA and the recently revised criteria.89 There is insufficient evidence to suggest causality as to whether the vaccine triggered such reactions, but the patient’s symptoms developed after receiving the COVID-19 vaccine (Pfizer-BioNTech), which led us to suspect an association with the vaccination.
However, the exact etiology of EGPA is not well understood. The disease is probably the result of a complex interaction between genetic and environmental factors that trigger an inflammatory response involving eosinophils and T and B lymphocytes.15 Environmental factors have been identified as triggers, including exposure to various allergens, infections, and vaccinations.11
In influenza vaccination, the same mRNA vaccine, like COVID-19 vaccination, the mechanism that causes the AAV is the molecular similarity between vaccine components and influenza antigen, which raises the possibility of activate the same autoimmune mechanisms.16 There are a few cases of new or recurrent AAV have been reported following influenza vaccination. In these patients, vasculitis developed 2–4 weeks after the vaccination.1718
The possible association between vaccines and autoimmune disorders may be attributed to several factors. Vaccine adjuvants, which are used to increase immune responses to vaccines, can lead to autoimmune responses.19 There is also the possibility that the influenza vaccine can activate the same autoimmune mechanisms as infectious antigens, owing to their structural similarities.20
A recent study reported that IMD flares or new-onset diseases are associated with COVID-19 vaccination. They found 27 cases in five large tertiary cohorts from three in Israel, one in the UK, and one in the USA, including 17 flares and 10 new-onsets.21 And a review of 29 cases of AAV found that 75% of the patients developed the condition following mRNA-based COVID-19 vaccines, with p-ANCA being the most frequently identified.22
So far it has been reported that only three patients have developed new-onset EGPA after receiving the mRNA-1273 (Moderna) or BNT162b2 vaccine. Among them, one patient experienced an exacerbation of pre-existing EGPA following BNT162b2 vaccination.62324
A possible limitation of this report could be that the causal relationship was defined mainly by the temporal relationship as clinically practiced for most cases of drug hypersensitivity. The other limitation could be that we do not know the possible underlying mechanisms such as which component of the COVID-19 vaccine may trigger. However, it is very important to recognize such a rare case of EGPA after the COVID-19 vaccination. As mentioned above, only three cases have been reported so far in the world. Further research is needed to collect more cases and to study underlying mechanisms.
Herein, we report the first case of EGPA after the administration of the COVID-19 vaccine in Korea. EGPA following vaccination is very rare, and the mechanism underlying its association with the disease is uncertain. Clinicians should be aware that EGPA can develop following COVID-19 vaccination.
Go to : 
References
1. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.

2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.

3. Kim BC, Kim HS, Han KH, Han SY, Jo HA. A case report of MPO-ANCA-associated vasculitis following heterologous mRNA1273 COVID-19 booster vaccination. J Korean Med Sci. 2022; 37(26):e204. PMID: 35790206.

4. Auanassova A, Yessirkepov M, Zimba O, Gasparyan AY, Joshi M, Agarwal V, et al. Physicians’ perceptions about antineutrophil cytoplasmic antibody-associated vasculitis: an online survey report in the time of the COVID-19 pandemic. Clin Rheumatol. 2023; 42(3):831–837. PMID: 36414862.

5. Watanabe T. vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 2017; 13(3):188–196. PMID: 28521688.

6. Ibrahim H, Alkhatib A, Meysami A. Eosinophilic granulomatosis with polyangiitis diagnosed in an elderly female after the second dose of mRNA vaccine against COVID-19. Cureus. 2022; 14(1):e21176. PMID: 35165624.

7. Gill R, Rizvi M, Sadiq MS, Feldman M. Recrudescence of severe polyneuropathy after receiving Pfizer-BioNTech COVID-19 vaccine in a patient with a history of eosinophilic granulomatosis with polyangiitis. BMJ Case Rep. 2022; 15(4):e245749.

8. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990; 33(8):1094–1100. PMID: 2202307.

9. Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, et al. 2022 American College of Rheumatology/European Alliance of Associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis. 2022; 81(3):309–314. PMID: 35110334.

10. Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. 2022; 28(7):542–554. PMID: 35537987.

11. Gioffredi A, Maritati F, Oliva E, Buzio C. Eosinophilic granulomatosis with polyangiitis: an overview. Front Immunol. 2014; 5:549. PMID: 25404930.

12. Mouthon L, Dunogue B, Guillevin L. Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (formerly named Churg-Strauss syndrome). J Autoimmun. 2014; 48-49:99–103. PMID: 24530234.

13. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013; 65(1):1–11. PMID: 23045170.

14. Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012; 130(3):607–612.e9. PMID: 22460074.

15. Vaglio A, Moosig F, Zwerina J. Churg-Strauss syndrome: update on pathophysiology and treatment. Curr Opin Rheumatol. 2012; 24(1):24–30. PMID: 22089097.
16. Duggal T, Segal P, Shah M, Carter-Monroe N, Manoharan P, Geetha D. Antineutrophil cytoplasmic antibody vasculitis associated with influenza vaccination. Am J Nephrol. 2013; 38(2):174–178. PMID: 23941822.

17. Birck R, Kaelsch I, Schnuelle P, Flores-Suárez LF, Nowack R. ANCA-associated vasculitis following influenza vaccination: causal association or mere coincidence? J Clin Rheumatol. 2009; 15(6):289–291. PMID: 19734734.
18. Spaetgens B, van Paassen P, Tervaert JW. Influenza vaccination in ANCA-associated vasculitis. Nephrol Dial Transplant. 2009; 24(10):3258. PMID: 19666908.

19. Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011; 36(1):4–8. PMID: 20708902.

20. Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003; 362(9396):1659–1666. PMID: 14630450.

21. Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel). 2021; 9(5):435. PMID: 33946748.

22. Prabhahar A, Naidu GS, Chauhan P, Sekar A, Sharma A, Sharma A, et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int. 2022; 42(4):749–758. PMID: 35124725.

23. Nappi E, De Santis M, Paoletti G, Pelaia C, Terenghi F, Pini D, et al. New onset of eosinophilic granulomatosis with polyangiitis following mRNA-based COVID-19 vaccine. Vaccines (Basel). 2022; 10(5):716. PMID: 35632472.

24. Chan-Chung C, Ong CS, Chan LL, Tan EK. Eosinophilic granulomatosis with polyangiitis after COVID-19 vaccination. QJM. 2022; 114(11):807–809. PMID: 34718799.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download