Written informed consent was obtained to publish this case report from the patient. The Institutional Review Board waived the review of this case report (IRB No. 2023-03-017).
A 34-year-old male (169.4 cm, 66.7 kg) presented to the hospital with persistent sputum for 2 months. He had no specific underlying disease and was not taking any medications. Chest computed tomography (CT) revealed multiple enlarged nodes in the mediastinum. We planned to perform EBUS under MAC, targeting moderate sedation that allows spontaneous ventilation in conjunction with a high-flow nasal cannula (HFNC). Before the procedure, the patient’s vital signs were stable, with a blood pressure of 119/83 mmHg, heart rate of 88 beats per min, respiratory rate of 20 breaths per min, oxygen saturation (SpO2) of 98%, and body temperature of 36.3°C. During the procedure, HFNC (Optiflow THRIVE, Fisher & Paykel Healthcare) was used to supply oxygen with a fraction of inspired oxygen (FiO2) of 1.0 and a flow of 40 L/min. Sedation was achieved with a bolus of remimazolam 5 mg and a target-controlled infusion of remifentanil at a target site concentration of 2.0 ng/ml. Before the procedure started, the patient’s sedation level was confirmed to be moderate. During the procedure, remimazolam was administered in bolus doses of 2.5 mg as needed (a total of 20 mg used) to maintain sedation depth, and remifentanil was adjusted to a range of 2.0–3.0 ng/ml.
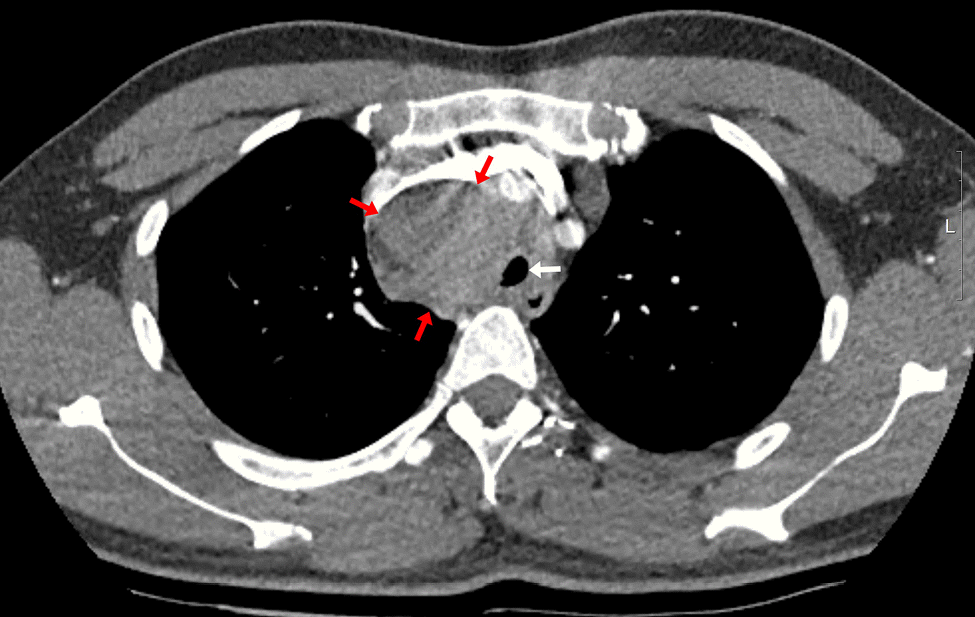
The patient had an enlarged lymph node (4.9 × 4.1 cm) in the right paratracheal area on chest CT, resulting in tracheal narrowing (7.3 mm) (
Fig. 1). No problems were encountered while inserting the flexible bronchoscope (BF-H290, Olympus), which had a 5.7 mm diameter, and no specific abnormalities were found in the entire bronchus. During the subsequent EBUS (BF-UC260FW, Olympus) insertion with a 6.9 mm diameter, mild resistance was encountered. The procedure started with bronchial suctioning for secretion removal to ensure a clear visual field. About 3 min after the insertion of the EBUS, the patient’s spontaneous breathing was maintained, but SpO
2 began to decrease below 90%. The procedure was stopped, and we applied a jaw thrust maneuver. However, as the saturation decreased, the EBUS bronchoscope was removed, and a jaw thrust was continued. However, the patient exhibited weak self-respiration, and SpO
2 rapidly decreased to approximately 30%. Bag-mask ventilation was immediately started, but the inspiratory pressure was high, and end-tidal CO
2 (EtCO
2) was also inconsistent, indicating inefficient ventilation. Within 1–2 min, SpO
2 slowly increased to 90%, but it did not increase further despite FiO
2 of 1.0. No secretions were observed on oral suction. We planned to reinsert the flexible bronchoscope to evaluate the cause of hypoxia. However, because SpO
2 was maintained at approximately 90% with positive-pressure mask ventilation, we thought it would be difficult to advance the bronchoscope with MAC, which has an insecure airway and depends on self-respiration. Therefore, we decided to proceed with bronchoscopy under mechanical ventilation after intubation. Because the biopsy site was not easily accessible after endotracheal tube (ETT) insertion owing to its location close to the vocal cord, the operator asked for a supraglottic airway device. However, the authors decided to use an ETT, considering the patient might require high positive airway pressure. To facilitate intubation, we administered an additional 50 mg of propofol and 40 mg of succinylcholine. After successful intubation with an ETT with an internal diameter of 7.5 mm, a large amount of pink frothy fluid was regurgitated through the ETT, and SpO
2 was restored to 95% after tracheal suction. Although continuous secretion was regurgitated, SpO
2 was maintained at approximately 90–95% with FiO
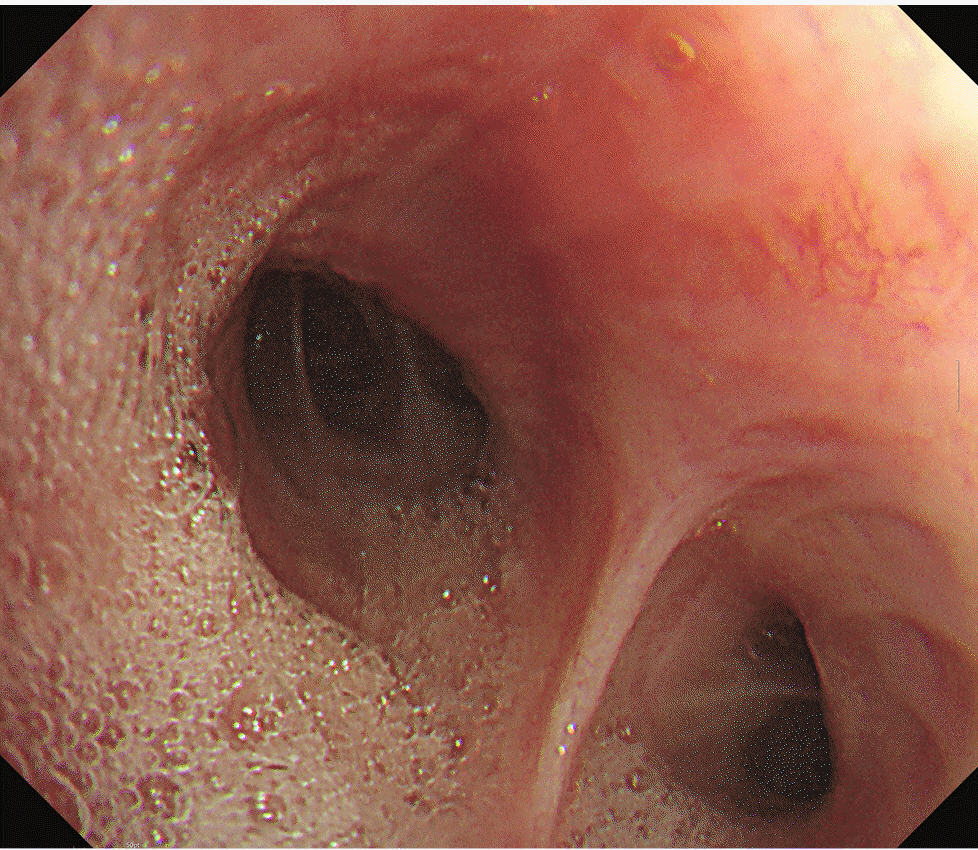
2 of 1.0. A bronchoscopic exam revealed a large amount of frothy fluid throughout the bronchi (
Fig. 2), and sufficient suction was performed. The patient could maintain SpO
2 without high positive-pressure ventilation after sufficient suction during bronchoscopy. The peak inspiratory pressure was reduced from 26 to 20 mmHg after bronchoscopic toileting of the secretion, at a tidal volume of 450 ml, respiratory rate of 14/min, and positive end-expiratory pressure (PEEP) of 5 cmH
2O. Subsequently, for the paratracheal lymph node biopsy, we administered propofol 20 mg and succinylcholine 30 mg, followed by the replacement of the ETT with a size 4 supraglottic airway device (i-gel, Intersurgical Ltd.). Biopsy using an EBUS bronchoscope was performed while maintaining positive-pressure ventilation through a supraglottic airway device. SpO
2 was maintained around 90% with FiO
2 of 1.0 for the duration of the procedure. After the procedure, the patient was extubated, and HFNC with FiO
2 of 1.0 and flow rate of 40 L was applied, maintaining SpO
2 above 90%. In the post-anesthesia care unit, the patient coughed severely and expectorated pink frothy sputum in a sitting position, but SpO
2 was maintained at approximately 95%. We gradually reduced the FiO
2 and flow rate to 0.75 and 30 L in 30 min while maintaining SpO
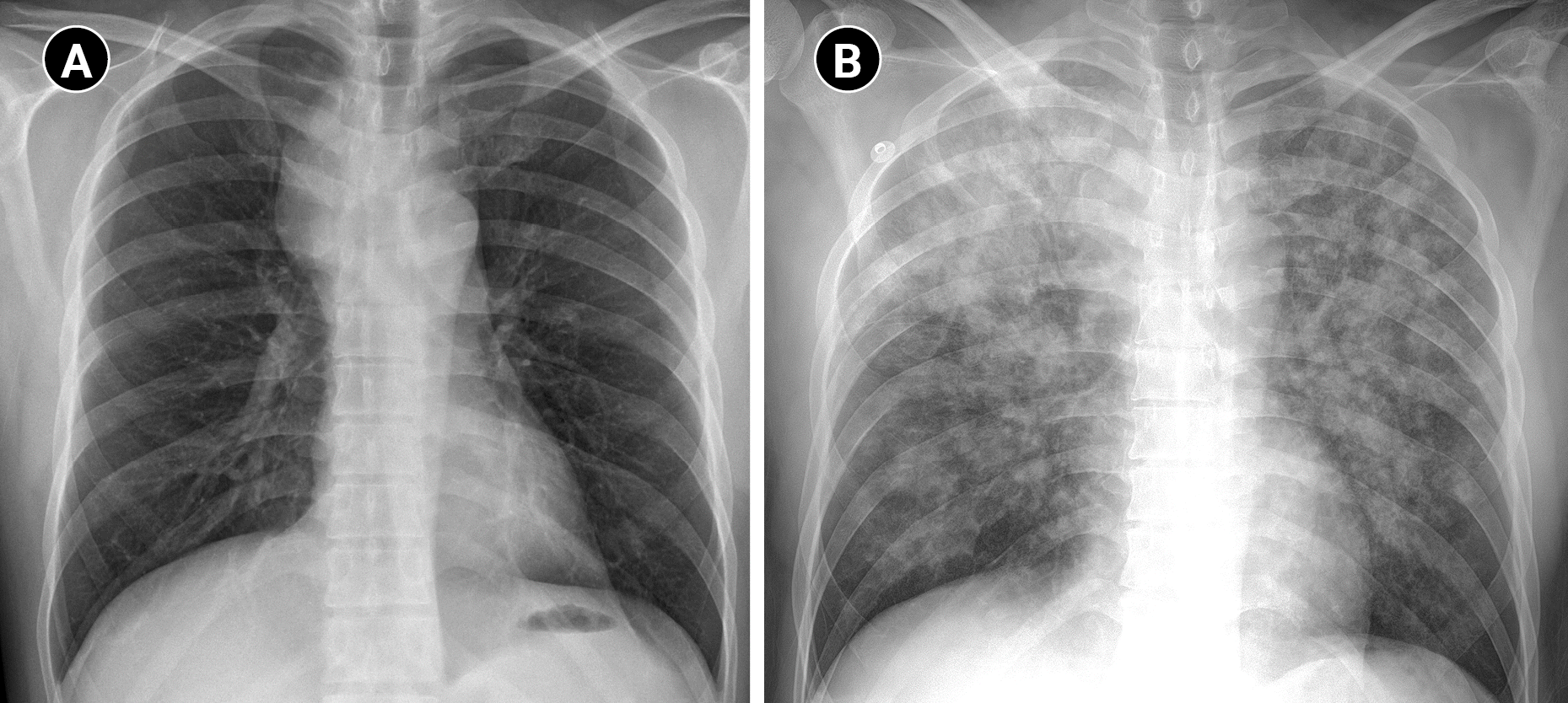
2 of 95%. The patient was transferred to the intensive care unit. Chest radiography after transfer showed bilateral pulmonary edema (
Fig. 3).
Overnight non-invasive ventilation was applied with inspiratory positive airway pressure of 11 cmH2O (above PEEP: 5 cmH2O), expiratory positive airway pressure of 6 cmH2O and FiO2 of 0.4 on the day of the procedure, and HFNC (FiO2 of 0.4 and a flow rate of 40 L/min) was maintained on postoperative day (POD) 1. On POD 2, the patient's SpO2 was above 90% using HFNC, so the patient was transferred to the general ward. The patient’s SpO2 was maintained at 98% in the general ward using HFNC and subsequently switched to nasal prongs with an oxygen flow rate of 3 L. The target SpO2 was 94%, and O2 was successfully tapered up to 1 L of oxygen. On POD 3, the patient's SpO2 was above 95% without supplemental O2. With no additional issues, the patient was discharged on POD 5.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download