Abstract
Background
Remifentanil and sufentanil are potent short-acting synthetic opioid analgesics. The administration of remifentanil has been associated with the incidence of opioid-induced hyperalgesia. Opioid-induced hyperalgesia may be alleviated when opioids, such as morphine, are switched to sufentanil. Therefore, this retrospective observational study aimed to compare the effects of remifentanil and sufentanil on postoperative pain in patients undergoing robotic gynecological surgery.
Methods
We retrospectively analyzed the electronic medical records of patients who underwent elective robotic gynecological surgery between January 2016 and February 2021. The patients were classified into sufentanil (n = 159) or remifentanil (n = 359) groups according to the opioids administered continuously during anesthesia. The primary outcome assessed in this study was the postoperative pain score measured using the numeric rating scale (NRS). The secondary outcomes assessed included the recovery time (from discontinuation of opioid infusion to extubation) and frequency of rescue analgesic administration in the post-anesthesia care unit (PACU).
Results
The recovery time did not differ significantly between the two groups. The NRS score for pain (median [1Q, 3Q]) in the PACU was significantly lower in the sufentanil group than in the remifentanil group (2 [2, 3] vs. 4 [3, 7], P < 0.001). The frequency of rescue analgesic administration in the PACU was 6.3% and 35.4% in the sufentanil and remifentanil groups, respectively (P < 0.001).
The use of nitrous oxide (N2O) in general anesthesia may lead to complications, such as the expansion of gas bubbles or air-containing space, diffusional hypoxia, megaloblastic anemia, and bone marrow suppression [1]. Therefore, continuously infused short-acting opioids are increasingly being used as adjuvants to volatile agents in general anesthesia as a substitute for N2O. Opioids reduce the dose of volatile or hypnotic agents without resulting in the expansion of bowel gas due to the use of N2O in robotic surgery requiring intra-abdominal CO2 gas insufflation. Robot-assisted laparoscopic surgery is a minimally invasive surgical approach and a standard alternative to open or laparoscopic gynecological surgery [2]. Robot-assisted laparoscopic surgery has all the advantages of robotic surgery, such as better visualization, more controlled and finer movements of the robotic arms enabling better dissection, and lesser blood loss; however, concerns regarding postoperative pain continue to pose a challenge.
Remifentanil is a short-acting synthetic opioid with a predictable and rapid recovery. It can be administered intraoperatively as an adjunct to inhalation or intravenous anesthesia. However, the administration of remifentanil has been associated with the incidence of opioid-induced hyperalgesia [3]. Although opioid-induced hyperalgesia has been consistently demonstrated in animal studies, the results of clinical trials in humans are controversial [4-7]. Some studies have reported that the intraoperative infusion of relatively high-dose remifentanil results in the development of opioid-induced hyperalgesia; in contrast, the infusion of low-dose remifentanil has shown no such effects [8-11].
Sufentanil, a highly selective μ-opioid receptor agonist, is more potent and has a longer duration of action than those of remifentanil [3,12]. In a previous study, opioid-induced hyperalgesia was alleviated when opioids, such as morphine, were switched to sufentanil [13]. Therefore, based on the pharmacological differences between sufentanil and remifentanil, we hypothesized that the postoperative pain of patients who received sufentanil would vary from that of those who received remifentanil as intraoperative anesthetic adjuvants. Thus, this study aimed to compare the effects of remifentanil and sufentanil on postoperative pain in patients undergoing robotic gynecological surgery.
This retrospective observational study was performed at a single university-affiliated hospital after obtaining approval from the appropriate Institutional Review Board (KYUH 2021-08-021). The requirement for obtaining informed consent from the participants was waived due to the retrospective nature of the study. This study was registered in the Clinical Research Information Service (https://cris.nih.go.kr/cris/index/index.do, registration number: KCT0007026).
We retrospectively analyzed the electronic medical records of patients who underwent elective robotic gynecological surgery between January 2016 and February 2021 at our hospital. Patients aged > 20 years with an American Society of Anesthesiologists physical status I or II were eligible for inclusion in this study. The exclusion criteria included colpopexies that caused severe pain and a history of psychiatric disorders or drug abuse.
The patients did not receive any sedative premedication. Anesthesia was induced using 1–2 mg/kg of propofol, and neuromuscular blockade was achieved using rocuronium or vecuronium. Sevoflurane (O2/air mixture: FiO2, 50%) and sufentanil- or remifentanil-target-controlled infusion (TCI) were used to maintain anesthesia. The same type of pump (Agilia® SP TIVA, Fresenius Kabi) was used for administering sufentanil and remifentanil infusions. The sufentanil and remifentanil pumps were programmed using the Gepts and Minto models, respectively [14]. The effect-site concentrations of sufentanil and remifentanil were maintained at 0.1 ng/ml (Gepts model) and 1 ng/ml (Minto model), respectively. The concentration of sevoflurane was adjusted to maintain a Patient State Index (PSI; SedLine®, Masimo Corp.) of 25–50. The choice of maintenance opioids was at the discretion of the anesthesiologist. The patients were classified into the sufentanil or remifentanil groups according to the opioid administered continuously during anesthesia. The administration of opioids was discontinued 10 min before the anticipated end of the surgery in both groups. Non-opioid analgesics were not administered intraoperatively. The administration of sevoflurane was discontinued at the end of the surgery, and 2–4 mg/kg of sugammadex was administered to reverse the neuromuscular block based on neuromuscular monitoring. Extubation was performed after confirming the adequacy of spontaneous respiration, response to verbal commands, and recovery of TOF ratio. All patients were transferred to the post-anesthesia care unit (PACU) at the end of the surgery, and postoperative pain was evaluated using a numerical rating scale (NRS; 0 = no pain, 10 = worst pain imaginable) for all patients upon arrival at the PACU. Analgesics, such as fentanyl, tramadol, and pethidine, were administered at the discretion of the attending anesthesiologist if the NRS score was ≥ 4 and the patient requested analgesics. All patients were observed in the PACU for at least 40 min according to the institutional protocol. The patients were observed for an additional 20 min from the time of drug administration if rescue drugs were administered due to pain or hemodynamic instability.
The following data were collected and analyzed: age; weight; height; duration of surgery and anesthesia; history of hypertension; American Society of Anesthesiologists physical status; and recovery profiles, such as recovery time from discontinuation of sufentanil or remifentanil to extubation in the operating room; NRS score for pain; frequency of rescue analgesic administration; and the incidence of respiratory complications in the PACU.
The primary outcome assessed in this study was the postoperative NRS score for pain. The secondary outcomes included recovery time in the operating room and the frequency of rescue analgesic administration in the PACU.
All statistical analyses were performed using SPSS version 27.0 (IBM Co.). After assessing the normality with the Shapiro–Wilk test, continuous variables were analyzed using the Student’s t-test or Mann–Whitney U test. Categorical variables were analyzed using the chi-square test or Fisher's exact test, as applicable. Data are expressed as mean ± standard deviation, number (%), or median (1Q, 3Q). Statistical significance was set at P < 0.05.
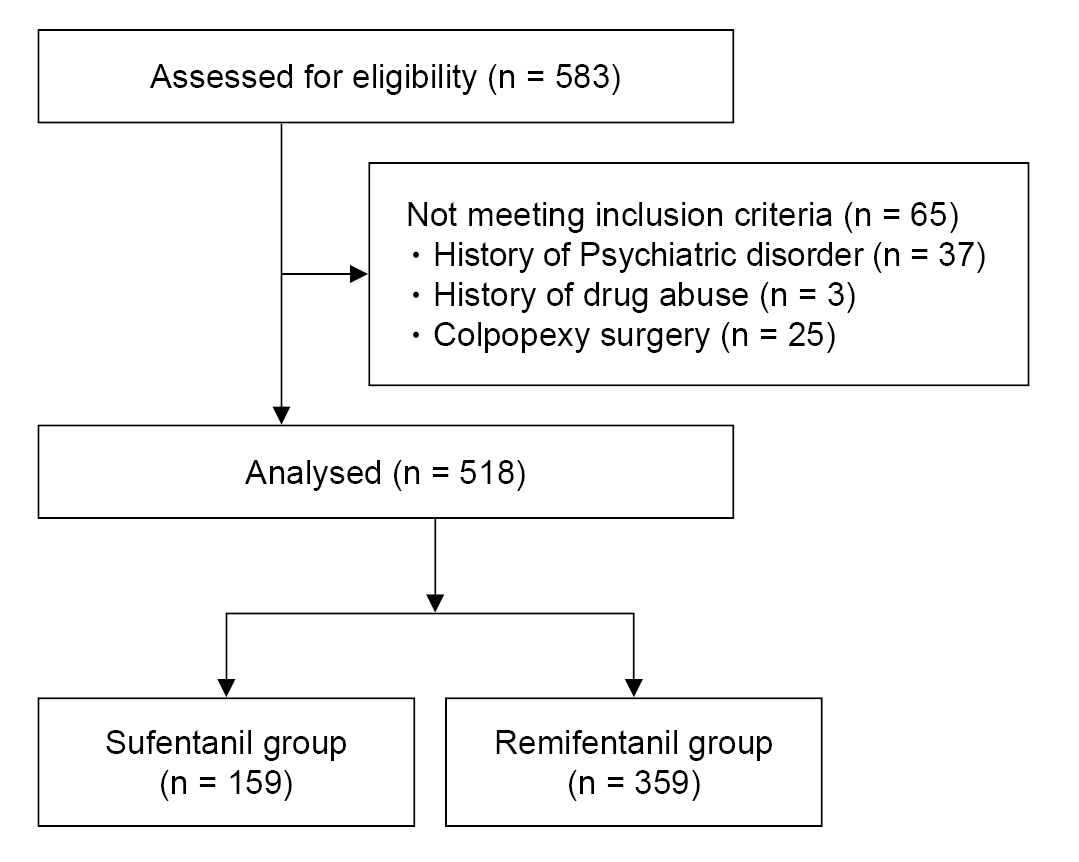
The electronic medical records of 583 patients who underwent elective robotic gynecological surgery between 2016 and February 2021 at our hospital were retrospectively analyzed. Sixty-five patients were excluded based on the exclusion criteria, and the remaining 518 patients were classified into the sufentanil (n = 159) or remifentanil (n = 359) groups (Fig. 1).
The demographic data did not differ significantly between the two groups (Table 1). Similarly, the blood pressure and heart rate also did not differ significantly between the two groups (Fig. 2).
Table 2 presents recovery profiles. The recovery time did not differ significantly between the two groups. However, the NRS pain score in the PACU was significantly lower in the sufentanil group than in the remifentanil group (2 [2–3] vs. 4 [3–7]; P < 0.001). Moreover, the number of patients requiring rescue analgesic administration for pain control in the PACU was significantly higher in the remifentanil group than in the sufentanil group (35.4% [127/359] vs. 6.3% [10/159]; odds ratio, 8.156; 95% confidence interval, [4.150–16.033]; P < 0.001).
Hypoxemia (SpO2 < 95%) accompanied by respiratory depression was observed in one patient in the sufentanil group in the PACU; however, the patient recovered rapidly following the application of an oxygen face mask and deep breathing.
The most important findings of the present study were that the NRS pain score and frequency of rescue analgesic administration were higher in the remifentanil group than those in the sufentanil group. The recovery time tended to be slightly longer in the sufentanil group than in the remifentanil group; however, the difference between the two groups was not significant. No hypoxemic events, except for one case in the sufentanil group, were observed in the two groups. Thus, the results of the present study suggest that sufentanil is superior to remifentanil in terms of postoperative pain management.
Although previous studies have evaluated the effects of intraoperative remifentanil and sufentanil infusion as anesthetic adjuvants on postoperative pain, to the best of our knowledge, this is the first study to be conducted on female patients who underwent robotic gynecological surgery. In this study, the median NRS pain scores were 2 and 4 in the sufentanil and remifentanil groups, respectively. The median difference between the two groups was only 2 points; however, it was considered clinically significant as an NRS score of ≥ 4 requires intervention for pain relief [15]. Postoperative rescue analgesics were administered at the discretion of the attending anesthesiologist according to the NRS scores and patient needs in the present study, resulting in a higher number of patients requiring additional rescue analgesics in the remifentanil group. The doses of sufentanil- and remifentanil-TCI were maintained at 0.1 ng/ml and 1.0 ng/ml, respectively, in the present study. As the potency of sufentanil is approximately 10 times greater than that of remifentanil [16], the doses of sufentanil and remifentanil used in this study correspond to equivalent doses.
Younger age; female sex; American Society of Anesthesiologists physical status I–II; the site of surgery, such as musculoskeletal or intra-abdominal surgery; and longer duration of surgery are known risk factors for postoperative pain [15]. Therefore, female patients with American Society of Anesthesiologists physical statuses I and II who underwent robot-assisted gynecological surgery and were expected to show no significant difference in the surgical site, invasiveness, duration of surgery, or age were included in this study to evaluate the effect of sufentanil- and remifentanil-TCI on postoperative pain. Sufentanil or remifentanil was administered as the adjuvant during general anesthesia at the anesthesiologist’s discretion.
Stress biomarkers, such as epinephrine, norepinephrine, cortisol, interleukin-6, and interleukin-10, showed no difference between the remifentanil and sufentanil groups 1 h after incision or at the end of surgery in patients anesthetized with remifentanil or sufentanil titrated to maintain hemodynamic parameters within 20% of the baseline combined with the same propofol regimen [17]. This result indicates that the inhibitory ability of stress hormones induced by the surgical simulation of remifentanil and sufentanil is similar when controlled according to the same hemodynamic target.
The effects of sufentanil and remifentanil on the postoperative pain score, analgesic requirements, and recovery time, when administered as adjuvants to volatile [14,18] or intravenous anesthetics [19-21], have been compared in various surgeries; however, the results have been inconsistent. As opioids used for fast-track cardiac anesthesia, sufentanil and remifentanil show no differences in postoperative pain management or time to excretion [1]. The pain score evaluated immediately after surgery was lower in the sufentanil group in patients undergoing laparoscopic cholecystectomy, but no difference in analgesic consumption was observed compared with the remifentanil group [19]. In contrast, remifentanil showed a faster extubation time than sufentanil under anesthesia for supratentorial craniotomy; however, there was no difference in the postoperative pain scores, and analgesic requirements within 1 h postoperatively were higher in the remifentanil group [20]. Sufentanil-TCI, which was used as an adjuvant to desflurane anesthesia in patients undergoing major abdominal therapy in a previous study, showed no difference in extubation time; however, the pain scores during the first 2 h after extubation were lower, and the cumulative morphine dose for pain control was significantly lower than that for remifentanil-TCI [14], which is similar to the results of our study. In addition, in a prospective multicenter study conducted on patients undergoing neurosurgery, sufentanil-propofol anesthesia resulted in no difference in the time to eye-opening and time to extubation; however, it reduced the analgesic requirements compared with remifentanil-propofol anesthesia [21].
The SpO2 values during the first 7 h postoperatively were lower in patients who received sufentanil-sevoflurane anesthesia than in those who received remifentanil-sevoflurane anesthesia in a previous study [22]. However, in other studies, when used as an adjunct to balanced [14] or total intravenous anesthesia [21], the use of sufentanil and remifentanil resulted in no differences in the incidence of respiratory complications. Similar to these results [14,21], respiratory complications occurred only in one patient in the sufentanil group, and the difference in the incidence of hypoxemia was not significant between the two groups in the present study. This may be attributed to the shorter operating time and lower sufentanil dosage used in this study. The average operative time did not exceed 2 h in either group in the present study. The context-sensitive half-life of sufentanil increases as the infusion duration increases; however, the half-life is approximately 20 min when infused for 2 h [23]. Sufentanil or remifentanil was discontinued 10 min before the anticipated end of the surgery, and the median time from opioid discontinuation to extubation was 15 min in both groups. In addition, compared with previous studies, sufentanil- and remifentanil-TCI were administered at fixed plasma concentrations of 0.1 ng/ml and 1 ng/ml, respectively, which are lower maintenance doses [14,17].
This study had some limitations. First, due to the retrospective nature of the study, all confounding factors that may have affected the outcomes could not be controlled. In addition, as propensity scoring methods were not applied, the results after matching pre- and intraoperative variables that may have affected the outcomes could not be confirmed. Second, unequal sample sizes may have led to unequal variances between the samples, which may have affected the statistical power and type 1 error rates.
In conclusion, sufentanil-sevoflurane anesthesia reduced the postoperative pain and the requirement for rescue analgesic administration compared with remifentanil-sevoflurane anesthesia in patients undergoing robot-assisted gynecological surgery. These findings suggest that sufentanil, as an adjunct to sevoflurane anesthesia is more advantageous than remifentanil in terms of postoperative pain control.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed in the current study are available from the corresponding author upon request.
AUTHOR CONTRIBUTIONS
Conceptualization: Young Seok Jee. Data curation: Inho Huh. Formal analysis: Tae-Yun Sung. Methodology: Young Seok Jee. Writing - original draft: Young Seok Jee. Writing - review & editing: Tae-Yun Sung, Sung-Ae Cho, Seok-Jin Lee, Choon-Kyu Cho. Investigation: Inho Huh. Supervision: Young Seok Jee.
REFERENCES
1. Becker DE, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008; 55:124–30.
2. Falcone T. Introduction: Robot-assisted laparoscopic surgery. Fertil Steril. 2014; 102:909–10.
3. Mandel JE. Considerations for the use of short-acting opioids in general anesthesia. J Clin Anesth. 2014; 26(1 Suppl):S1–7.

4. Gustorff B, Nahlik G, Hoerauf KH, Kress HG. The absence of acute tolerance during remifentanil infusion in volunteers. Anesth Analg. 2002; 94:1223–8.

5. Lahtinen P, Kokki H, Hynynen M. Remifentanil infusion does not induce opioid tolerance after cardiac surgery. J Cardiothorac Vasc Anesth. 2008; 22:225–9.

6. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998; 86:1307–11.

7. Rauf K, Vohra A, Fernandez-Jimenez P, O'Keeffe N, Forrest M. Remifentanil infusion in association with fentanyl-propofol anaesthesia in patients undergoing cardiac surgery: effects on morphine requirement and postoperative analgesia. Br J Anaesth. 2005; 95:611–5.

8. Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000; 93:409–17.
9. Schmidt S, Bethge C, Förster MH, Schäfer M. Enhanced postoperative sensitivity to painful pressure stimulation after intraoperative high dose remifentanil in patients without significant surgical site pain. Clin J Pain. 2007; 23:605–11.

10. Shin SW, Cho AR, Lee HJ, Kim HJ, Byeon GJ, Yoon JW, et al. Maintenance anaesthetics during remifentanil-based anaesthesia might affect postoperative pain control after breast cancer surgery. Br J Anaesth. 2010; 105:661–7.

11. Yeom JH, Kim KH, Chon MS, Byun JW, Cho SY. Remifentanil used as adjuvant in general anesthesia for spinal fusion does not exhibit acute opioid tolerance. Korean J Anesthesiol. 2012; 63:103–7.

12. Glass PSA, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999; 89(4 Suppl):S7.

13. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006; 104:570–87.

14. Derrode N, Lebrun F, Levron JC, Chauvin M, Debaene B. Influence of peroperative opioid on postoperative pain after major abdominal surgery: sufentanil TCI versus remifentanil TCI. A randomized, controlled study. Br J Anaesth. 2003; 91:842–9.

15. Mei W, Seeling M, Franck M, Radtke F, Brantner B, Wernecke KD, et al. Independent risk factors for postoperative pain in need of intervention early after awakening from general anaesthesia. Eur J Pain. 2010; 14:149. e1-7.

16. Glass PSA, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999; 89(4 Suppl):7.

17. Liu YH, Hu XB, Yang XM, Wang YW, Deng M. Comparing remifentanil and sufentanil in stress reduction during neurosurgery: a randomised controlled trial. Int J Clin Pharm. 2020; 42:1326–34.

18. Engoren M, Luther G, Fenn-Buderer N. A comparison of fentanyl, sufentanil, and remifentanil for fast-track cardiac anesthesia. Anesth Analg. 2001; 93:859–64.

19. Damen SL, Nieuwenhuijs VB, Joosten W, Houweling PL, Clevers GJ. The effects of remifentanil and sufentanil on the quality of recovery after day case laparoscopic cholecystectomy: a randomized blinded trial. J Laparoendosc Adv Surg Tech A. 2004; 14:87–92.

20. Gerlach K, Uhlig T, Hüppe M, Nowak G, Schmitz A, Saager L, et al. Remifentanil-propofol versus sufentanil-propofol anaesthesia for supratentorial craniotomy: a randomized trial. Eur J Anaesthesiol. 2003; 20:813–20.

21. Martorano PP, Aloj F, Baietta S, Fiorelli A, Munari M, Paccagnella F, et al. Sufentanil-propofol vs remifentanil-propofol during total intravenous anesthesia for neurosurgery. A multicentre study. Minerva Anestesiol. 2008; 74:233–43.
22. Casati A, Albertin A, Fanelli G, Deni F, Berti M, Danelli G, et al. A comparison of remifentanil and sufentanil as adjuvants during sevoflurane anesthesia with epidural analgesia for upper abdominal surgery: effects on postoperative recovery and respiratory function. Anesth Analg. 2000; 91:1269–73.

Table 1.
Demographic Data
Table 2.
Recovery Profiles




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download