Abstract
The purpose of perioperative fluid management in children is to maintain adequate volume status, electrolyte level, and endocrine system homeostasis during the perioperative period. Although hypotonic solutions containing glucose have traditionally been used as pediatric maintenance fluids, recent studies have shown that isotonic balanced crystalloid solutions lower the risk of hyponatremia and metabolic acidosis perioperatively. Isotonic balanced solutions have been found to exhibit safer and more physiologically appropriate characteristics for perioperative fluid maintenance and replacement. Additionally, adding 1%–2.5% glucose to the maintenance fluid can help prevent children from developing hypoglycemia as well as lipid mobilization, ketosis, and hyperglycemia. The fasting time should be as short as possible without compromising safety; recent guidelines have recommended that the duration of clear fluid fasting be reduced to 1 h. The ongoing loss of fluid and blood as well as the free water retention induced by antidiuretic hormone secretion are unique characteristics of postoperative fluid management that must be considered. Reducing the infusion rate of the isotonic balanced solution may be necessary to avoid dilutional hyponatremia during the postoperative period. In summary, perioperative fluid management in pediatric patients requires careful attention because of the limited reserve capacity in this population. Isotonic balanced solutions appear to be the safest and most beneficial choice for most pediatric patients, considering their physiology and safety concerns.
The first intravenous (IV) fluid used in humans was administered to resuscitate a patient dying from malignant cholera [1]. At the earliest stages of IV fluid development, the goal of fluid management was simply to replace intravascular volume loss to recover from hypovolemia. However, hypervolemia was soon found to be as dangerous as hypovolemia. Disturbances in body volume or electrolyte balance can result in impaired organ function and unfavorable outcomes, such as mortality [2,3]. The goal of perioperative fluid management is to maintain homeostasis and central euvolemia and prevent excess salt and water accumulation [4]. To attain a normal physiological state, maintaining or re-establishing extracellular fluid (ECF) volume, blood volume, tissue perfusion, metabolic function, electrolyte balance, and an appropriate acid-base status is necessary [5]. To achieve these goals, Holiday and Segar’s formula, commonly called the “4-2-1” rule, has been used to calculate the infusion rate of maintenance fluids. However, the “4-2-1” rule was based on a study that only included healthy persons in non-perioperative settings (Table 1) [6]. Additionally, current pediatric anesthesia clinical practice is considerably different from that in Holiday and Segar’s era. Today, we have a better understanding of pediatric physiology, including organ maturation, perioperative fluid and electrolyte requirements, and the effects of preoperative fasting. This review covers the basic terminology, fluid management principles, and fluid physiology relevant to children and the history of IV fluids, appropriate type and volume of intraoperative IV fluids, preoperative fasting management, and the impact of hormonal changes on postoperative fluid management.
We begin with the basic terminology used to describe electrolyte solutions’ effect on water movement into and out of the cell.
The osmotic concentration, commonly called “osmolarity,” is a measurement of the osmotic activity of electrolyte solutions. Osmolarity is the number of osmoles of solute per volume of solution (Osm/L). Homeostasis is the ability of an organism to maintain a stable internal environment. In humans, this involves the dynamic balance of electrolytes and water in the intracellular fluid (ICF) and ECF, which includes plasma. Disruption of this balance can lead to dehydration, edema, acidosis, alkalosis, and changes in the plasma electrolyte concentrations (e.g., hypo- or hypernatremia).
The osmolarity of a specific solution can be measured using an osmometer or calculated from the composition of the solutes. However, a simple summation of the osmolarity of all solutes in a solution (theoretical osmolarity) is not equal to the measured value (real osmolarity). The osmolarity can be calculated using the following equation [7]:
Real osmolarity = Theoretical osmolarity × Osmotic coefficient
For example, a 0.9% Sodium(Na) chloride(Cl) solution has a theoretical osmolarity of 308 mOsm/L (154 mOsm/L Na + 154 mOsm/L Cl), and the osmotic coefficient of the 0.9% NaCl solution is 0.93. If we substitute these numbers in the above equation, we find that the real osmolarity of 0.9% NaCl is 286 mOsm/L (308 mOsm/L × 0.93) [7].
The term “osmolality” is used to express osmotic concentration. To calculate the osmolality, the mass of the solvent is used instead of the volume to define the osmotic concentration. This can be described as the number of osmoles of solute per unit mass of solution, which is Osm/kg H2O in most solutions for IV use.
The term “tonicity” is used to describe the behavior of a particular solution when a specific cell is fully submerged. The solution is considered hypotonic if the cell swells, with a net movement of water from the solution into the cell, and hypertonic if the cell shrinks, with a net movement of water out of the cell. Isotonic solutions do not alter the cell volume.
Osmolarity and tonicity are different concepts. While both are used to compare the concentration of solutes between two solutions separated by a semipermeable membrane (e.g., the membrane of a human cell), the two terms differ in the definition of effective solutes. Cells can absorb some electrolytes through the cell membrane via specialized transport proteins, which helps to prevent the electrolytes from functioning as osmotically effective solutes in vivo [8]. While both permeable and nonpermeable solutes are considered effective for osmolarity, only nonpermeable solutes are considered effective for tonicity. Consequently, an isotonic solution for one species may not be isotonic for another. For example, in the 19th century, scientific societies held the false belief that a 0.6% NaCl solution was isotonic to humans based on experimental data from frogs [9].
The actual osmolarity of human plasma is 288 mOsmol/kg H2O, which is not significantly different from the theoretical osmolarity of 291 mOsmol/L. Therefore, a solution is considered isotonic to human cells if the sum of the concentrations of the nonpermeable solutes is not significantly different from 290 mOsmol/L.
For example, using an osmometer, the osmolarity of a 0.9% NaCl solution is 286 mOsmol/L. Because both Na and Cl are nonpermeable solutes to human cell membranes, 0.9% NaCl is considered isosmotic and isotonic to human plasma. It is important to note that an isosmotic solution is not always isotonic but can also be hypotonic. For example, although the actual osmolarity of a 5% dextrose water solution (5DW) is 278 mOsmol/L, it is considered hypotonic since, unlike Na or Cl, glucose can completely permeate human cell membranes. When glucose enters the cell, it drags water along with it via osmosis, causing the cell to expand in volume. Additionally, glucose metabolizes into energy, carbon dioxide, and water once inside the cell, so 5DW is not different from pure water in terms of tonicity [8].
To summarize, a hypoosmotic solution is always hypotonic, whereas an isosmotic solution can be either hypotonic or isotonic. Hyperosmotic solutions can be hypotonic, isotonic, or hypertonic. For example, a 10% dextrose water (10DW) solution is considered both hyperosmotic and hypotonic in humans.
Now we will discuss how fluid and electrolytes are balanced in children.
The two-compartment model, which consists of the ECF and ICF, is commonly used to describe body fluids. The ECF to ICF ratio changes continuously as a child grows. The proportion of the ECF to the ICF volume is higher in the fetus (45% vs. 35% of body weight). By 1 month of age, however, the ECF and ICF volumes are equivalent, and the ratio of the ECF to the ICF as seen in adults (1 : 2) is reached by the age of 1–3 years. Given that infants have a relatively high ECF compared to ICF volume, they are more susceptible to dehydration and fluid imbalance.
The proportion of total body fluids to body weight decreases with age. In the fetus, the proportion is as high as 80%, falling to 70% at full term, 60% by the age of 1 year, and 50%–60% after puberty [10]. This decrease in water is primarily due to a decrease in the ECF volume as the ICF volume increases with cell growth. Fortunately, the composition of the ECF, including plasma, is consistent across all ages, which allows for the same electrolyte-containing fluids to be used in adults and children if their kidneys have matured and can appropriately handle the electrolyte concentrations and water volume. The ECF consists of three compartments: the plasma, interstitial fluid, and transcellular fluid. Only 25% of the ECF is plasma; the rest is interstitial fluid. Transcellular fluid consists of the lymph, cerebrospinal fluid, aqueous and vitreous humor, synovial fluid, and serous fluid. The volume of transcelluar fluid is clinically negligible in healthy children (Fig. 1) [11].
Na and Cl are the major electrolytes in the ECF, including in the plasma. The concentrations of potassium (K), phosphate, magnesium, and proteins are higher in the ICF than in the ECF. The composition of the interstitial fluid is similar to that of the plasma, except for lower levels of proteins in the interstitial fluid [12]. This uneven distribution of fluids and electrolytes between the ECF and ICF is mediated by the sodium-potassium adenosine triphosphatase pump (Na-K ATP pump) in the cell membrane (cations) and the Gibbs–Donnan effect (anions). The Na-K ATP pump enables Na and K to be the major cations in the ECF and ICF, respectively. The Gibbs–Donnan effect is a phenomenon of uneven distribution of diffusible ions between a semipermeable membrane owing to the presence of non-diffusible ions. In body fluids, negatively charged proteins in the ICF cannot cross cellular membranes, making Cl a major anion in the ECF [13].
Electrolyte levels depend on the dynamic equilibrium between intake and output. In healthy children, electrolyte loss occurs primarily through the urine, followed by the skin. A total of 2–3 mEq/kg Na, 1–2 mEq/kg K, and 5 mEq/kg Cl leave the body in urine every day. The loss of Na and K through the skin is much lower (0.5 mEq/kg per day). To compensate for this loss, the daily electrolyte requirements should be as follows: 3 mEq/kg Na, 2 mEq/kg K, and 5 mEq/kg Cl [6,14].
In 1628, William Harvey first explained the closed circulation of blood in the human body in his famous book, “Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus,” translated as “An Anatomical Exercise on the Motion of the Heart and Blood in Living Beings.” However, the first successful report of plasma replacement appeared approximately 200 years later, after the second cholera pandemic in England [1]. Dr. Thomas Latta was the first to successfully treat cholera with an IV fluid injection in 1832. He wrote “I at length resolved to throw the fluid immediately into the circulation. In this, having no precedent to direct me, I proceeded with much caution.” Of the initial four patients who received IV fluids, one patient survived. He attributed the low success rate to the low volumes infused, administration at a late stage of the disease process, and underlying diseases [1]. Latta’s initial IV fluid was a mixture of “two drachms of muriate, and two scruples of carbonate of soda, to sixty ounces of water,” making it a hypotonic and hyponatremic solution of NaCl and sodium carbonate mixed in water. The concentration was as follows: Na 106 mmol/L, Cl 78 mmol/L, and carbonate 14 mmol/L [15]. This hypotonic solution would induce hemolysis and may thus be related to the high rate of resuscitation failure. Subsequently, Latta modified the solution to be closer to the concentrations in plasma, at 134 mmol/L Na, 118 mmol/L Cl, and 16 mmol/L bicarbonate [16]. However, IV fluid resuscitation did not gain popularity after the sudden death of Latta from pulmonary tuberculosis and the end of the cholera pandemic.
Nearly 50 years passed before a similar physiologic IV solution, designed by Sydney Ringer, appeared in the literature in 1882. Ringer’s solution maintained the rhythmicity of frog cardiac muscle in ex vivo studies better than saline, although he believed that 0.6% NaCl was isotonic to human serum, based on frog experiments [17]. In 1888, “normal saline” first appeared in a printed article; however, the composition was far from equivalent to that of the ECF: 150 mmol Na, 128 mmol Cl, 2.5 mmol phosphate, and 27 mmol bicarbonate in 1,000 ml of water [18]. In 1921, Hartog Hamburger published a report that human blood and NaCl 0.9% solution were isotonic, based on a freezing point comparison. The in vitro study conducted by Hamburger provided the first scientific support for the use of a 0.9% NaCl solution [19]. In 1924, Rudolph Matas advocated “the value and advantages of the IV route for the direct and continuous instillation of fluids intended to replace the volume of lost blood,” prompting a new era of continuous IV drips [20]. In 1932, the American pediatrician Alexis Hartmann replaced the unstable bicarbonate in Ringer’s solution with stable lactate while treating children with metabolic acidosis due to severe diarrhea, emphasizing the advantage of a balanced salt solution [21]. Calcium-free acetate-buffered isotonic solutions (e.g., PlasmaLyte) have recently been introduced into clinical practice. Although a better physiological profile has been associated with a balanced salt solution, even in the 1930s, the 0.9% NaCl solution is still widely used perioperatively [22].
Pediatric intraoperative fluid management involves four major considerations. The first is tonicity, which is primarily determined by Na concentration. The second is the use of a balanced solution, with buffered solutions containing less Cl than unbalanced or unbuffered solutions. The third is glucose levels, which are associated with hypoglycemia, lipolysis, ketosis, or even shock if depleted and hyperglycemia if excessive. The fourth is the use of a colloid solution containing protein or starch to maintain intravascular oncotic pressure. Many medical societies have developed guidelines and recommendations to address these issues.
In this section, we will use the terms hyponatremic and isonatremic instead of hypotonic and isotonic to increase understanding of the historical context of hypotonic maintenance fluid solutions in pediatrics.
Holiday and Segar calculated the basal fluid and electrolyte requirements for children. The Na requirement calculated was 3 mEq/100 cal/day [6]. If a child’s body weight is 5 kg, the amount of fluid calculated using the “4-2-1” rule is 4 (cc/h/kg) × 5 (kg) × 24 (h/day) = 480 ml/day. Because the “4-2-1” rule is based on the observation that processing one calory requires one milliliter of water, the daily requirement of Na is 3 (mEq/100 cal/day) × 480 (ml/cal) = 14.4 mEq/day. For a child with complete oral fasting and minimal movement, 480 ml of water and 14.4 mEq of Na should be supplied daily; an IV fluid solution containing 30 mEq/L Na (14.4 mEq/0.48 L = 30 mEq/L) can be used. This is the same Na concentration as that of 0.18% NaCl with 4% glucose (154 mEq/L × 0.2 = 31 mEq/L), which is only 21.4% of the Na concentration of human plasma (140 mEq/L × 0.214 = 30 mEq/L). This is the rationale for using 0.18% NaCl with 4% glucose as the maintenance solution for neonates and infants; however, this is actually a hyponatremic solution. In the 1960s and early 1970s, severe hyponatremic fluids, such as 0.2% NaCl or even 5% dextrose water (which contains no Na) was used as a maintenance and replacement fluid perioperatively because of the false belief that children’s immature kidneys could not excrete Na properly. Although subsequent studies have demonstrated that the concentration function of the kidney matures rapidly, reaching 80–90% of adult levels by six months of age, the universal practice of administering hyponatremic solutions during anesthesia persisted. Because Na is the primary determinant of the osmolarity of a solution, a hyponatremic solution is hypotonic in most cases. When administered over a short period, such severely hypotonic fluids can induce acute cerebral edema and, ultimately, brain herniation due to the net movement of water. Prepubescent children are more vulnerable to hyponatremia-induced brain edema than adults because of their increased brain-size-to-cranial-vault ratio, decreased Na-K ATPase activity, and increased antidiuretic hormone (ADH) levels in response to stress [23,24]. Children who receive hyponatremic fluids may experience increased irritability, headaches, seizures, and even sudden death [24,25]. In 2014, a Cochrane review compared isotonic and hypotonic solutions as maintenance IV fluids in children. This review included ten studies with 1,106 total patients. Most patients were admitted to an intensive care setting. The risk of hyponatremia was decreased by 52% in the isotonic solution group [26]. This finding is consistent with that of a well-designed double-blind randomized controlled trial (RCT) comparing an isotonic IV fluid containing 140 mmol/L Na to a hypotonic solution with 77 mmol/L Na as the IV maintenance fluid in children. The group that received the isotonic fluid had a lower risk of hyponatremia, with no increase in adverse effects [27].
Even in the 1960s, Holliday was also aware of the risk of hyponatremia associated with large volumes of hypotonic solution [28,29]. Although he recommended isotonic solutions for volume expansion or compensation for volume deficits, he still preferred hypotonic solutions for maintenance [30]. However, considering practical concerns, such as the need for additional IV lines and situations requiring rapid volume expansion, isotonic fluids are more appropriate for intraoperative use.
Although ‘normal’ is too broad and vague of a term, we call 0.9% NaCl a ‘normal’ saline solution. Unfortunately, no scientific background supports the use of the word ‘normal’ for the 0.9% NaCl solution, which consists of 9 g NaCl in 1,000 ml of water. Although this solution is isotonic to human blood, this does not mean that it is normal or physiologic [31].
Moreover, the term ‘normal’ in normal saline gives a false sense of security, making it dangerous since the human response to a 0.9% NaCl solution may not be benign. Even in healthy volunteers, a large volume infusion of 0.9% NaCl has been associated with abdominal discomfort, pain, nausea, drowsiness, and decreased mental capacity to perform complex tasks [32]. Hyperchloremia, metabolic acidosis, fluid retention, renal vascular constriction, and reduced glomerular filtration rate can also occur in both adults and children following a 0.9% NaCl infusion [34–37]. Additionally, this solution can cause cellular dysfunction by inducing cytosolic acidification, membrane hyperpolarization, inactivation of protein kinases, and disruption of phosphorylation [38]. Although the 0.9% NaCl solution is isotonic to human plasma, it can induce pathological changes in humans. Therefore, caution is advised in calling this solution ‘normal.’
A balanced crystalloid solution (BCS) is physiologically more similar to human plasma than the 0.9% NaCl solution. A BCS contains lactate, acetate, or malate as a bicarbonate precursor to prevent hyperchloremic and dilutional metabolic acidosis, which is observed after 0.9% NaCl infusions. Compared to lactate, acetate metabolism is significantly faster and more independent of hepatic function, with a lower increase in oxygen consumption and no interference with the diagnostic use of lactate as a marker of inadequate tissue perfusion [8]. A BCS can be used to normalize electrolyte imbalance, maintain homeostasis, and provide a margin of safety in cases of accidental hyperinfusion [39]. In a well-designed RCT of critically ill adults, the use of a BCS was associated with a lower rate of death and new renal replacement therapy than the use of a 0.9% NaCl solution [40]. In adult patients with sepsis, the beneficial effect of a BCS on mortality was greater than that of saline when fluid choice was controlled earlier [41]. Among non-critically ill adults, the incidence of major adverse kidney events within 30 days was lower with a BCS than with 0.9% NaCl [42]. A recent meta-analysis of 13 studies showed that the BCS was associated with lower hospital or 28–30 day mortality in critically ill adults [43]. However, the mortality and acute kidney injury rates did not differ between the BCS and 0.9% NaCl solution groups in a Cochrane review of critically ill adult and pediatric patients. However, it should be noticed that, only 258 pediatric patients were included in this Cochrane review [44].
A recent meta-analysis of three RCTs with 162 critically ill pediatric patients showed that metabolic acidosis and bicarbonate levels improved after 4–12 h of hydration with a BCS compared with a 0.9% NaCl solution [45]. The European Society of Pediatric and Neonatal Intensive Care recently conducted a systematic review and published recommendations for IV maintenance fluid therapy. In solid consensus, isotonic BCS was recommended as a maintenance fluid in acute and critically ill children [46]. The European consensus statement in 2011 and guidelines from the Association of the Scientific Medical Societies in Germany in 2016 also recommend an isotonic BCS be used for intraoperative maintenance fluid therapy in children [5,47].
During surgery, surgical stress increases plasma counter-insulin hormone levels (e.g., cortisol, glucagon, epinephrine, and growth hormone) and decreases plasma insulin levels, which leads to a hyperglycemia-induced catabolic state [48]. However, if the glucose supplement is insufficient (e.g., long preoperative fasting with no glucose supplement during surgery), lipolysis and ketogenesis occur after depletion of glycogen and gluconeogenetic substrates (e.g., alanine from skeletal muscle) [49]. This leads to a lower or lower-normal glucose concentration, elevated levels of ketone bodies and free fatty acids, reduced base excess, and the occurrence of ketoacidosis [50].
To prevent hypoglycemia and catabolic reactions, fluids containing 5% glucose (5DS) have gained popularity as a maintenance infusion for children [23]. However, as the glucose concentration of 5DS is 5,000 mg/dl, which is approximately 50 times higher than that in plasma, perioperative infusion of 5DS can induce hyperglycemia [50].
In pediatric patients, both hypoglycemia and hyperglycemia are associated with neuronal damage [51]. To minimize the risk of glucose and endocrine homeostasis disruption (hyperglycemia, hypoglycemia, lipid mobilization, and ketosis), recent consensus guidelines recommend 1%–2.5% glucose-containing solutions be used as perioperative maintenance fluids for children [47,52].
However, the amount of glucose administered should be individualized, and the anesthesiologist should monitor plasma glucose levels regularly. By adhering to the recommended preoperative fasting time for a healthy child past the neonatal stage, the administration of a glucose-free solution is unlikely to disrupt glucose and lipid homeostasis during brief (< 1 h), minimally traumatic surgery such as inguinal hernia repair [53]. In fact, the incidence of preoperative hypoglycemia is between 0% and 2.5% and is usually associated with a longer duration of fasting. Accordingly, routine administration of glucose is not necessary in healthy children. In contrast, for a child at a high risk of hypoglycemia, a 2.5% glucose-containing solution may not be sufficient to prevent perioperative hypoglycemia and ketosis [54]. Children in a catabolic state and/or with a low glycogen reserve (e.g., long fasting time, burns, prematurity, debilitation, malnourishment, and liver disease) are at a higher risk of perioperative hypoglycemia. One RCT found that a 2%–4% DS administered at a rate of 10 ml/kg/h was more effective at preventing intraoperative catabolism, insulin resistance, rebound hyperglycemia, and acidosis than a 1% DS in low-birth- weight neonates [55].
The use of colloids in clinical practice is relatively new in medical history. The first case series of IV human albumin (HA) use was published in 1941 for severely burned and injured sailors during World War II [56]. Albumin is an essential component of human plasma proteins. The human liver synthesizes 10–12 g of albumin, which is degraded spontaneously daily. The half-life of albumin in human plasma is up to 3 weeks [57]. Albumin comprises more than 50% of plasma proteins and is responsible for 80% of the intravascular oncotic pressure [58]. Besides maintaining oncotic plasma pressure, albumin increases plasma concentrations of thiols, which are essential antioxidants of the ECF [59], modulates the activity of nitric oxide by generating an S-nitroso adduct of serum albumin [60], and acts as a buffer for hydrogen ions [61]. However, few studies have shown that external albumin administration has a clinical impact on these mechanisms.
The use of HA in clinical settings remains controversial. HA administration has not been found to decrease mortality in critically ill adults [62–64]. The actual intravascular volume expansion efficacy of albumin in clinical settings, when compared with that of a crystalloid solution, is often much less than theoretically expected. The theoretical volume expansion efficacy of albumin can be achieved under the conditions of an intact vascular barrier and normal permeability. However, critical illness and inflammatory responses are frequently associated with the degradation of the endothelial glycocalyx layer and increased vascular permeability, which facilitate water and solute leakage into the interstitium. This could partly explain the lack of clinical benefit associated with HA in critically ill patients with edema or sepsis.
HA is the scarcest and most expensive colloid besides plasma. Additionally, HA has a high associated risk of infection. Therefore, since the 1970s, synthetic colloids such as the hydroxyethyl starch solution (HES) have gradually replaced HA in clinical practice [65].
The HES is a corn- or potato-based starch containing 0.9% saline or balanced crystalloids. Each HES has a unique molecular weight, degree of substitution, C2/C6 ratio, and concentration. The third-generation HES has a lower molecular weight of 130,000 Da and shows an improved safety profile with a lower risk of renal failure and pruritus and fewer hemostatic alterations, while maintaining the same volume effects [66,67]. The third -generation HES in a balanced electrolyte solution showed fewer acid-base and electrolyte alterations than 0.9% saline [68].
However, the use of a HES in adults, especially under critical conditions, has been controversial. A recent large meta-analysis showed that the HES is associated with an increased risk of blood transfusions and renal replacement therapy in critically ill patients. However, immediate 30- and 90-day mortality rates did not differ significantly [69]. In another meta-analysis, compared with a low-molecular-weight (third-generation) HES in patients with septic shock, a first- or second-generation HES was associated with a significant risk of acute kidney injury and renal replacement therapy, whereas the third-generation HES was associated with an increased risk of renal replacement therapy but not acute kidney injury [70].
In pediatric patients undergoing surgery, a meta-analysis of nine RCTs showed that perioperative volume expansion with a third-generation HES did not alter renal function, blood loss, or blood transfusions [71]. The intraoperative use of a 6% HES 130/0.4 up to 30 ml/kg was not associated with postoperative acute kidney injury in pediatric cardiac patients [72]. However, HES products are under regulatory suspension for all ages in Europe and the USA [73,74].
The most favored intraoperative maintenance fluid is an isotonic BCS with 1%–2.5% glucose. This solution helps to maintain electrolyte and endocrine homeostasis in children during surgery. The “4-2-1” rule, along with the additional fluid requirements based on the invasiveness of the surgical procedure (2 ml/kg/h for minor, 4 mg/kg/h for intermediate, and 6 ml/kg/h for major trauma) is still useful for calculating intraoperative maintenance in children. Anesthesiologists should carefully monitor the ongoing blood and fluid loss and compensate for this loss with an isotonic BCS free of glucose or blood, as necessary. Individualized volume replacement helps optimize the cardiac output based on dynamic variables specific to pediatric patients and their vital signs [75]. Colloids can be added to compensate for volume loss and to maintain the plasma oncotic pressure.
The fasting time associated with volume deficits influences intraoperative fluid management. Prolonged fasting is associated with patient discomfort, nausea and vomiting, thirst, hunger, anxiety, and metabolic changes, including ketoacidosis. However, these adverse effects are less likely to occur when the traditional “6-4-2” fasting guideline is strictly followed [76]. Excessive fasting is common, with some patients fasting for up to 15 h, which is much longer than the recommended fasting time of 2 h [77,78]. Recent studies have suggested that shorter clear-fluid fasting durations may be more beneficial. Two well-designed multidisciplinary approaches that included educating parents and medical personnel and encouraging clear fluid intake even up to 2 h before surgery, failed to reduce the clear fluid fasting time. In contrast, after changing the minimum fasting time to 1 h, prolonged fasting decreased by more than 50% [79,80]. The residual volume of the stomach and gastric pH did not differ between the 1-h and 2-h clear fluid fasting groups [81]. Pooled data and audits of liberal fluid intake guidelines have shown that clear fluid in the stomach of children during the induction of elective anesthesia does not increase the risk of aspiration [82].
In 2022, the European Society of Anesthesiology and Intensive Care published updated guidelines for preoperative fasting in children in which the 6 (solid, infant formula) - 4 (breast milk) - 2 (clear fluid) regimen was changed to the 6 (solid) - 4 (infant formula) - 3 (breast milk) - 1 (clear fluid) regimen (each number representing the minimal hours of fasting) [83]. This reduction in the recommended preoperative clear fluid fasting time is consistent with the 2018 consensus statement from the European Society for Pediatric Anesthesiology (ESPA) [84]. Although this minimum recommended clear-fluid fasting time may be controversial, achieving the shortest possible fasting time without compromising patient safety is essential. The ESPA recommends ≤ 3 ml/kg of clear fluid based on a study examining serial magnetic resonance imaging, which showed that the residual gastric volume returned to baseline 1 h after ingestion of 3 ml/kg sugared clear fluid [85].
Children should start drinking fluids as early as possible after anesthesia. However, timing should be based on the child’s urge to drink. Forced postoperative drinking is associated with increased vomiting [83]. When early oral intake is not possible or insufficient, IV fluid support is essential to maintain normovolemia. Postoperative IV fluid management involves two unique aspects: ongoing body fluid loss and free water retention. Table 2 shows the composition of body fluids. Most body fluids are isotonic; however, they can be hyponatremic or isonatremic. Using a hyponatremic solution as a postoperative maintenance fluid is associated with a high risk of iatrogenic hyponatremia.
Free water retention following surgery is another cause of iatrogenic hyponatremia. Renin and ADH are released to retain salt and water in response to perioperative hypovolemia. Renin promotes Na and water retention via aldosterone, while ADH induces water resorption via the water channels of the collecting tubules and ducts in the kidneys. Intravascular volume depletion is the most potent stimulus of ADH release. Although this reaction is physiological and thus not inappropriate, various osmotic and non-osmotic factors, including pain, inflammation, surgical stress, hypoxia, hypercapnia, sepsis, organ dysfunction, and drugs potentiate the release of excessive amounts of ADH, causing the syndrome of inappropriate antidiuretic hormone secretion (SIADH) to occur [86]. Children in the postoperative period are at risk of dilutional hyponatremia.
To compensate for body fluid loss and free water retention and to minimize the risk of iatrogenic hyponatremia, the postoperative maintenance solution should be isotonic and isonatremic [27]. If a patient has a risk of water retention associated with ADH secretion, restricting fluids to 50%–80% of routine maintenance can be considered.
Even in 1972, Holliday recommended that the maintenance fluid infusion rate be reduced to half of the “4-2-1” rule when the urine output is decreased due to ADH secretion [87]. In 2007, he modified his maintenance fluid therapy recommendations for acutely ill and mild-to-moderately hypovolemic children with ADH secretion. He recommended that 20–40 ml/kg of isotonic solution be administered rapidly within 1–2 h to stop the secretion of ADH and that hypotonic solution be administered per his original recommendation, with the rate reduced by half of the “4-2-1” rule [88]. However, he was a pediatrician and not a pediatric anesthesiologist, and his recommendation was not intended for the postoperative state. As previously mentioned, because of other ADH-stimulating factors, recovery from hypovolemia is not sufficient to stop the secretion of ADH during and after surgery.
Thus, during the postoperative period, the fluid management priority is early oral fluid intake based on ameliorating thirst. If parenteral fluid management is necessary, isotonic solutions should be used as maintenance fluids in children, and the rate should be half of the “4-2-1” rule (i.e., the “2-1-0.5” rule) when hourly urine volume is diminished due to ADH secretion. After urine volume is normalized, the rate may be increased to again follow the “4-2-1” rule (Table 1).
For perioperative fluid management, anesthesiologists should be aware of the perioperative pathophysiology of children and the characteristics of the fluids (Table 3). Excessive preoperative fasting is associated with discomfort, dehydration, and a catabolic state. The preoperative fasting time should be kept to a minimum to ensure patient safety. An isotonic balanced solution shows better physiological characteristics and is safer for perioperative maintenance and replacement than other fluids. For intraoperative maintenance, adding 1%–2.5% glucose can help prevent glucose and endocrine homeostasis disruption in some patients. During the postoperative period, children should be encouraged to drink fluids when thirsty. The rate of postoperative fluid administration should be adjusted to account for the effects of free water retention by renin and ADH as well as ongoing fluid and blood loss during the postoperative period.
Notes
References
1. Lewins R. Injection of saline in extraordinary quantities into the veins in cases of malignant cholera. Lancet. 1832; 18:243–4.
3. Arieff AI, Ayus JC, Fraser CL. Hyponatraemia and death or permanent brain damage in healthy children. BMJ. 1992; 304:1218–22.

4. Miller TE, Myles PS. Perioperative fluid therapy for major surgery. Anesthesiology. 2019; 130:825–32.

5. Sümpelmann R, Becke K, Brenner S, Breschan C, Eich C, Höhne C, et al. Perioperative intravenous fluid therapy in children: guidelines from the Association of the Scientific Medical Societies in Germany. Paediatr Anaesth. 2017; 27:10–8.

6. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957; 19:823–32.

7. Sümpelmann R, Becke K, Zander R, Witt L. Perioperative fluid management in children: can we sum it all up now? Curr Opin Anaesthesiol. 2019; 32:384–91.
8. Zander R. Infusion fluids: why should they be balanced solutions? EJHP Pract. 2006; 12:60–2.
9. Lazarus-Barlow WS. On the initial rate of osmosis of blood-serum with reference to the composition of "Physiological Saline Solution" in mammals. J Physiol. 1896; 20:145–57.

11. Friis-Hansen B. Body water compartments in children: changes during growth and related changes in body composition. Pediatrics. 1961; 28:169–81.

12. Hill LL. Body composition, normal electrolyte concentrations, and the maintenance of normal volume, tonicity, and acid-base metabolism. Pediatr Clin North Am. 1990; 37:241–56.

13. Donnan FG. Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. J Memb Sci. 1995; 100:45–55.

14. Boineau FG, Lewy JE. Estimation of parenteral fluid requirements. Pediatr Clin North Am. 1990; 37:257–64.

15. Latta T. Malignant cholera. Documents communicated by the Central Board of Health, London, relative to the treatment of cholera by the copious injection of aqueous and saline fluids into the veins. Lancet. 1832; 18:274–80.
17. Latta T. Saline venous injection in cases of malignant cholera, performed while in the vapour-bath. Part II. Lancet. 1832; 19:208–9.
18. Ringer S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol. 1883; 4:29–42.

19. Churton T. Leeds General Infirmary: a case of scirrhus of the pylorus, with excessive vomiting; repeated intravenous injections of saline solution; remarks. Lancet. 1888; 132:620–1.
20. Hamburger HJ. A discourse on permeability in physiology and pathology. Lancet. 1921; 198:1039–45.

21. Matas R. The continued intravenous "DRIP": with remarks on the value of continued gastric drainage and irrigation by nasal intubation with a gastroduodenal tube (JUTTE) in surgical practice. Ann Surg. 1924; 79:643–61.
22. Hartmann AF, Senn MJ. Studies in the metabolism of sodium r-lactate. III. Response of human subjects with liver damage, disturbed water and mineral balance, and renal insufficiency to the intravenous injection of sodium r-lactate. J Clin Invest. 1932; 11:345–55.

23. Way C, Dhamrait R, Wade A, Walker I. Perioperative fluid therapy in children: a survey of current prescribing practice. Br J Anaesth. 2006; 97:371–9.
24. Ayus JC, Achinger SG, Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol. 2008; 295:F619–24.

25. Arieff AI. Postoperative hyponatraemic encephalopathy following elective surgery in children. Paediatr Anaesth. 1998; 8:1–4.

26. Mai CL, Yaster M, Chu L, Zulfiqar A, Firth PG. The development of pediatric fluid resuscitation: an interview with Dr. Frederic A. 'Fritz' Berry. Paediatr Anaesth. 2014; 24:217–23.
27. McNab S, Ware RS, Neville KA, Choong K, Coulthard MG, Duke T, et al. Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. Cochrane Database Syst Rev. 2014; (12):CD009457.

28. McNab S, Duke T, South M, Babl FE, Lee KJ, Arnup SJ, et al. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial. Lancet. 2015; 385:1190–7.

29. Holliday MA. Water, and salt and water: a distinction should be made. Pediatrics. 1965; 36:821–4.

30. Dugan S, Holliday MA. Water intoxication in two infants following the voluntary ingestion of excessive fluids. Pediatrics. 1967; 39:418–20.

31. Mann C, Held U, Herzog S, Baenziger O. Impact of normal saline infusion on postoperative metabolic acidosis. Paediatr Anaesth. 2009; 19:1070–7.

32. Holliday MA, Ray PE, Friedman AL. Fluid therapy for children: facts, fashions and questions. Arch Dis Child. 2007; 92:546–50.

33. Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999; 88:999–1003.

34. Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond). 2003; 104:17–24.

36. Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999; 90:1265–70.

37. Hoorn EJ. Intravenous fluids: balancing solutions. J Nephrol. 2017; 30:485–492. Erratum in: J Nephrol 2020; 33: 387.

38. Cotton BA, Guy JS, Morris JA Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006; 26:115–21.

39. Witt L, Osthaus WA, Lücke T, Jüttner B, Teich N, Jänisch S, et al. Safety of glucose-containing solutions during accidental hyperinfusion in piglets. Br J Anaesth. 2010; 105:635–9.

40. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018; 378:829–39.

41. Jackson KE, Wang L, Casey JD, Bernard GR, Self WH, Rice TW, et al. Effect of early balanced crystalloids before ICU admission on sepsis outcomes. Chest. 2021; 159:585–95.

42. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018; 378:819–28.

43. Hammond DA, Lam SW, Rech MA, Smith MN, Westrick J, Trivedi AP, et al. Balanced crystalloids versus saline in critically ill adults: a systematic review and meta-analysis. Ann Pharmacother. 2020; 54:5–13.

44. Antequera Martín AM, Barea Mendoza JA, Muriel A, Sáez I, Chico-Fernández M, Estrada-Lorenzo JM, et al. Buffered solutions versus 0.9% saline for resuscitation in critically ill adults and children. Cochrane Database Syst Rev. 2019; 7:CD012247.

45. Lehr AR, Rached-d’Astous S, Barrowman N, Tsampalieros A, Parker M, McIntyre L, et al. Balanced versus unbalanced fluid in critically ill children: systematic review and meta-analysis. Pediatr Crit Care Med. 2022; 23:181–91.

46. Brossier DW, Tume LN, Briant AR, Chaparro CJ, Moullet C, Rooze S, et al. ESPNIC clinical practice guidelines: intravenous maintenance fluid therapy in acute and critically ill children—a systematic review and meta-analysis. Intensive Care Med. 2022; 48:1691–1708.

47. Sümpelmann R, Becke K, Crean P, Jöhr M, Lönnqvist PA, Strauss JM, et al. European consensus statement for intraoperative fluid therapy in children. Eur J Anaesthesiol. 2011; 28:637–9.

48. Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology. 2008; 108:506–23.
49. Sawada A, Kamada Y, Hayashi H, Ichinose H, Sumita S, Yamakage M. Effect of intraoperative glucose infusion on catabolism of adipose tissue and muscle protein in patients anesthetized with remifentanil in combination with sevoflurane during major surgery: a randomized controlled multicenter trial. Anesth Analg. 2016; 123:869–76.

50. Nishina K, Mikawa K, Maekawa N, Asano M, Obara H. Effects of exogenous intravenous glucose on plasma glucose and lipid homeostasis in anesthetized infants. Anesthesiology. 1995; 83:258–63.

51. Malone JI. Diabetic central neuropathy: CNS damage related to hyperglycemia. Diabetes. 2016; 65:355–7.

52. Becke K, Eich C, Höhne C, Jöhr M, Machotta A, Schreiber M, et al. Choosing wisely in pediatric anesthesia: an interpretation from the German Scientific Working Group of Paediatric Anaesthesia (WAKKA). Paediatr Anaesth. 2018; 28:588–96.
53. Mikawa K, Maekawa N, Goto R, Tanaka O, Yaku H, Obara H. Effects of exogenous intravenous glucose on plasma glucose and lipid homeostasis in anesthetized children. Anesthesiology. 1991; 74:1017–22.

54. Neville KA, Sandeman DJ, Rubinstein A, Henry GM, McGlynn M, Walker JL. Prevention of hyponatremia during maintenance intravenous fluid administration: a prospective randomized study of fluid type versus fluid rate. J Pediatr. 2010; 156:313–9.

55. Datta PK, Pawar DK, Baidya DK, Maitra S, Aravindan A, Srinivas M, et al. Dextrose-containing intraoperative fluid in neonates: a randomized controlled trial. Paediatr Anaesth. 2016; 26:599–607.

56. Caironi P, Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009; 7:259–67.
57. Peters T Jr. All about albumin: biochemistry, genetics, and medical applications. San Diego: Academic Press;1995.
58. Weil MH, Henning RJ, Puri VK. Colloid oncotic pressure: clinical significance. Crit Care Med. 1979; 7:113–6.
60. Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, et al. Nitric oxide circulates in mammalian plasma primarily as an s-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992; 89:7674–7.

61. Reeves RB. Temperature-induced changes in blood acid-base status: donnan rCl and red cell volume. J Appl Physiol. 1976; 40:762–7.

62. Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998; 317:235–40.
63. Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2011; 2011:CD001208.

64. Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus. 2013; 11 Suppl 4(Suppl 4):s18–25.
65. Huskisson L. Intravenous volume replacement: which fluid and why? Arch Dis Child. 1992; 67:649–53.

66. Joosten A, Coeckelenbergh S, Alexander B, Delaporte A, Cannesson M, Duranteau J, et al. Hydroxyethyl starch for perioperative goal-directed fluid therapy in 2020: a narrative review. BMC Anesthesiol. 2020; 20:209.

67. Westphal M, James MF, Kozek-Langenecker S, Stocker R, Guidet B, Van Aken H. Hydroxyethyl starches: different products--different effects. Anesthesiology. 2009; 111:187–202.
68. Sümpelmann R, Witt L, Brütt M, Osterkorn D, Koppert W, Osthaus WA. Changes in acid-base, electrolyte and hemoglobin concentrations during infusion of hydroxyethyl starch 130/0.42/6 : 1 in normal saline or in balanced electrolyte solution in children. Paediatr Anaesth. 2010; 20:100–4.
69. Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018; 8:CD000567.

70. Li B, Zhao H, Zhang J, Yan Q, Li T, Liu L. Resuscitation fluids in septic shock: a network meta-analysis of randomized controlled trials. Shock. 2020; 53:679–85.

71. Thy M, Montmayeur J, Julien-Marsollier F, Michelet D, Brasher C, Dahmani S, et al. Safety and efficacy of peri-operative administration of hydroxyethyl starch in children undergoing surgery: a systematic review and meta-analysis. Eur J Anaesthesiol. 2018; 35:484–95.
72. Oh HW, Lee JH, Kim HC, Kim EH, Song IK, Kim HS, et al. The effect of 6% hydroxyethyl starch (130/0.4) on acute kidney injury in paediatric cardiac surgery: a prospective, randomised trial. Anaesthesia. 2018; 73:205–15.

73. U.S. Food & Drug Administration. Labeling changes on mortality, kidney injury, and excess bleeding with hydroxyethyl starch products [Internet]. Silver Spring (MD): Food and Drug Administration; 2021 July 7 [cited 2023 Feb 19]. Available from www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/labeling-changes-mortality-kidney-injury-and-excess-bleeding-hydroxyethyl-starch-products.
74. European Medicines Agency. Hydroxyethyl starch (HES) solutions for infusion: suspension of marketing authorisations due to continued use in contraindicated patient populations with increased risk of serious harm [Internet]. Amsterdam: European Medicines Agency; 2022 June 30 [updated 2022 Jul 26; cited 2023 Feb 19]. Available from www.ema.europa.eu/en/medicines/dhpc/hydroxyethyl-starch-hes-solutions-infusion-suspension-marketing-authorisations-due-continued-use.
75. Lee JH, Kim EH, Jang YE, Kim HS, Kim JT. Fluid responsiveness in the pediatric population. Korean J Anesthesiol. 2019; 72:429–40. Erratum in: Korean J Anesthesiol 2019; 72: 624. Erratum in: Korean J Anesthesiol 2021; 74: 188.

76. Disma N, Frykholm P, Cook-Sather SD, Lerman J. Pro-con debate: 1- vs 2-hour fast for clear liquids before anesthesia in children. Anesth Analg. 2021; 133:581–91.

77. Buller Y, Sims C. Prolonged fasting of children before anaesthesia is common in private practice. Anaesth Intensive Care. 2016; 44:107–10.

78. Engelhardt T, Wilson G, Horne L, Weiss M, Schmitz A. Are you hungry? Are you thirsty?--fasting times in elective outpatient pediatric patients. Paediatr Anaesth. 2011; 21:964–8.
79. Newton RJG, Stuart GM, Willdridge DJ, Thomas M. Using quality improvement methods to reduce clear fluid fasting times in children on a preoperative ward. Paediatr Anaesth. 2017; 27:793–800.

80. Isserman R, Elliott E, Subramanyam R, Kraus B, Sutherland T, Madu C, et al. Quality improvement project to reduce pediatric clear liquid fasting times prior to anesthesia. Paediatr Anaesth. 2019; 29:698–704.

81. Schmidt AR, Buehler P, Seglias L, Stark T, Brotschi B, Renner T, et al. Gastric pH and residual volume after 1 and 2 h fasting time for clear fluids in children†. Br J Anaesth. 2015; 114:477–82.

82. Frykholm P, Disma N, Kranke P, Afshari A. The rationale for the recommendations of the European paediatric fasting guideline: improving paediatric anaesthesia and perioperative medicine. Eur J Anaesthesiol. 2022; 39:1–3.

83. Frykholm P, Disma N, Andersson H, Beck C, Bouvet L, Cercueil E, et al. Pre-operative fasting in children: a guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol. 2022; 39:4–25.
84. Thomas M, Morrison C, Newton R, Schindler E. Consensus statement on clear fluids fasting for elective pediatric general anesthesia. Paediatr Anaesth. 2018; 28:411–4.

85. Schmitz A, Kellenberger CJ, Liamlahi R, Studhalter M, Weiss M. Gastric emptying after overnight fasting and clear fluid intake: a prospective investigation using serial magnetic resonance imaging in healthy children. Br J Anaesth. 2011; 107:425–9.

86. Cote CJ, Lerman J, Anderson B. A practice of anesthesia for infants and children. 6th ed. Philadelphia: Elsevier;2017. p. 199–216.
87. Holliday MA. Body fluid physiology during growth. In: Maxwell & Kleeman's Clinical Disorders of Fluid and Electrolyte Metabolism. 2nd ed. Narins RG, editor. New York: McGraw‐Hill;1972.
Fig. 1.
Distribution of body water in a (A) 30-kg child aged 9 years and (B) 4.5-kg child aged 1 month. The height of a graph is proportional to the volume. ECF: extracellular fluid, ICF: intracellular fluid, TBW: total body water.
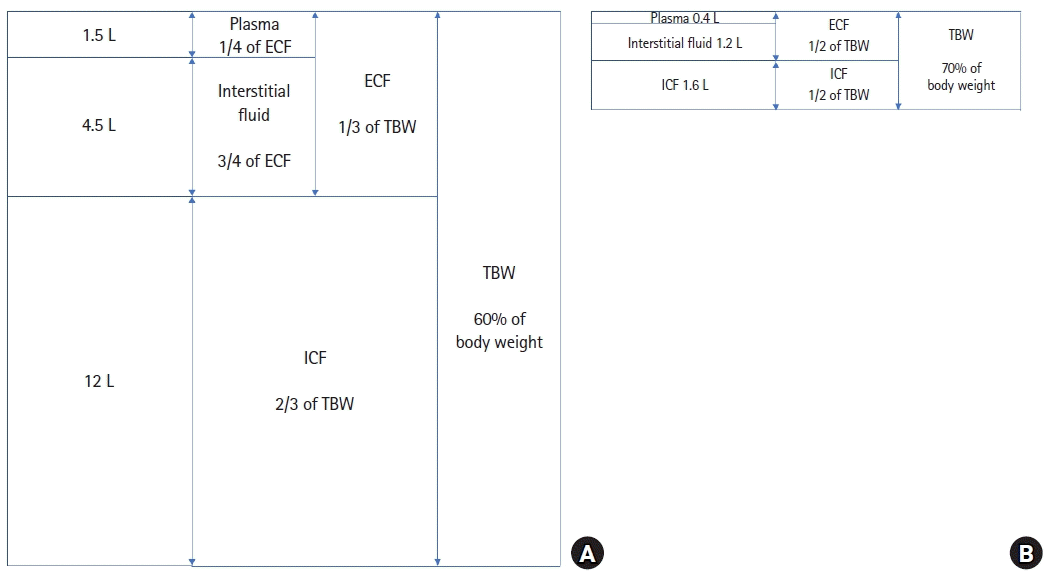
Table 1.
The “4-2-1” and “2-1-0.5” Rules
Table 2.
Composition of Body Fluids
Table 3.
Composition of Plasma and Crystalloid Fluids




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download