Abstract
Background
The primary aim of this study was to investigate the final adult height (FAH) of girls diagnosed with central precocious puberty (CPP) who were untreated.
Methods
We retrospectively analyzed the medical records of 36 girls diagnosed with CPP between 8 and 9 years of age who did not receive treatment, and 206 girls diagnosed with CPP within the same age range who received gonadotropin-releasing hormone (GnRH) agonist treatment. Midparental height (MPH), predicted adult height (PAH) obtained using height and bone age (BA) at the time of diagnosis (PAH for BA), and PAH obtained using the Bayley-Pinneau method (PAH by BP) were calculated. Additionally, height at the time of growth completion was compared with the predicted height.
Results
The FAHs were 160.71±4.56 cm in the untreated group and 159.31±4.26 cm in the treated group. In the untreated group, the FAH was 0.99±4.50 cm shorter than the MPH but 4.29±3.33 cm and 3.46±3.93 cm greater than the PAH for BA and PAH by BP, respectively.
Conclusion
In children diagnosed with CPP between 8 and 9 years of age who were untreated, FAH was greater than PAH for BA and PAH by BP at the time of diagnosis, indicating that the prognosis of FAH was not poor. Therefore, for girls diagnosed with CPP, it is recommended to consider various conditions, such as pubertal onset, height at diagnosis, BA, peak luteinizing hormone level, predicted height, and speed of puberty, when deciding whether to administer GnRH agonists.
Central precocious puberty (CPP) has idiopathic or organic causes and refers to the onset of secondary sexual characteristics due to the early activation of the hypothalamic-pituitary-gonadal (HPG) axis in girls and boys before 8 and 9 years of age, respectively [1,2]. Early puberty accelerates growth and bone maturation, resulting in premature union, which reduces final adult height (FAH) [3,4]. Thus, preserving adult height is one of the main reasons for considering treatment of CPP with gonadotropin-releasing hormone (GnRH) agonists, which downregulate the HPG axis and limit pubertal progression [5].
However, the therapeutic effect of GnRH agonists on adult height varies depending on the progression of CPP, which has transient, slowly progressive, and rapidly progressive forms depending on the level of puberty progression. It is difficult to distinguish between the slowly and rapidly progressive forms [6]. If puberty progresses rapidly, treatment is required; however, if puberty progresses slowly, the prognosis for FAH is good even without treatment [7].
In Korea, treatment is often initiated late in children diagnosed with CPP, between 8 and 9 years of age for girls and between 9 and 10 years of age for boys, according to the reimbursement criteria for GnRH agonist treatment. However, the effect of initiating treatment at these ages on FAH is unclear [8]. There have been no studies on FAH in untreated patients because most children receive treatment.
Therefore, this study aimed to (1) investigate the FAH and onset of menstruation in girls diagnosed with CPP between 8 and 9 years of age who visited the Department of Pediatric Endocrinology at Pusan National University Hospital but did not receive treatment; (2) compare the FAH with the predicted adult height (PAH) at the time of diagnosis; and (3) compare the FAH between girls with CPP without treatment and those with CPP who initiated GnRH agonist treatment at 8 to 9 years of age.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Pusan National University Hospital, Busan, Korea (IRB No: 2207-004-116). Informed consent was obtained from the parents of all patients.
We retrospectively analyzed the medical records of girls diagnosed with CPP. Thirty-six girls diagnosed with CPP between 8 and 9 years of age at the Department of Pediatric Endocrinology, Pusan National University Hospital from March 2013 until April 2022, who were not treated with GnRH agonists, were classified into the untreated group. Two hundred and six girls diagnosed with CPP at the same hospital from March 2013 until February 2021, who initiated GnRH agonist treatment between 8 and 9 years of age, were categorized into the treated group.
CPP was diagnosed based on breast development before 8 years of age, faster bone age (BA) than chronological age (CA), and a maximum luteinizing hormone (LH) value of ≥5 IU/L with an increase of more than twice the baseline value, according to the GnRH stimulation test. Patients who had received growth hormone therapy and those with other chronic diseases, including thyroid disease, were excluded if there was an organic cause of disease.
All children in the treated group were treated with 90 µg/kg of leuprolide acetate or triptorelin acetate every 4 weeks until CA of 11 years or BA of 12 to 12.5 years.
We collected data on the patients’ clinical characteristics such as age, height, weight, body mass index (BMI), BA, LH concentration, midparental height (MPH), and Tanner stage at the time of CPP diagnosis. BA was determined according to the Greulich and Pyle atlas, and breast development was evaluated according to Tanner staging.
The MPH was defined as the average of the parental heights minus 6.5 cm. The standard deviation scores (SDSs) for height, weight, and BMI according to the age at diagnosis were calculated according to the 2017 Korean National Growth Chart for Children and Adolescents enacted by the Korean Pediatric Society. The height SDS for BA was calculated by replacing CA with BA in the growth chart. The predicted adult height obtained using height and bone age at the time of diagnosis (PAH for BA) was calculated by replacing the height SDS of BA in the growth chart with the height of an 18-year-old to 11-month-old girl. The value calculated by the Bayley-Pinneau method (BP) for the expected adult height was defined as the PAH by BP. We used an average table if the BA was within 1 year of the CA and an accelerated table if the BA was more than 1 year older than the chronological age.
The parents of each pediatric patient were informed of the current study at the time the patient reached late adolescence or was expected to reach her FAH, and the FAH and age at the onset of menarche were investigated. The FAH was determined when the growth rate reached <2 cm/year. We compared FAH, MPH, PAH for BA, and PAH by BP at the time of diagnosis and compared the results obtained with and without treatment.
All data are expressed as mean±standard deviation. Continuous variables were analyzed using independent t-tests to compare the untreated and treated groups. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using R software ver. 4.1.3 (http://cran.r-project.org).
Clinical characteristics such as CA, BA, the gap between BA and CA, height, height SDS for BA, weight, BMI, MPH, PAH for BA, and PAH by BP were compared between the untreated and treated groups at the time of CPP diagnosis (Table 1). The mean pubertal onset ages were 7.92±0.63 and 7.88±0.32 years in the untreated and treated groups, respectively, but there was no significant difference between the two groups (p=0.674). The mean CAs were 8.34±0.74 and 8.60±0.30 years in the untreated and treated groups, respectively. Patients in the untreated group were younger than those in the treated group (p=0.044). The mean BAs in the untreated and treated groups were 9.98±0.89 and 10.44±0.75 years, respectively, and the BA was younger in the untreated group than in the treated group (p=0.005). In the groups, BA progressed by approximately 1.64±0.91 and 1.84±0.67 years, respectively, compared to CA, but the gap between BA and CA was not significantly different between the two groups (p=0.116). The height SDSs for BA were –0.98±0.94 and –1.32±0.79 in the untreated and treated groups, respectively, indicating that patients were significantly taller in the untreated group than in the treated group (p=0.043). Body weights were 30.21±5.56 kg and 32.68±5.91 kg in the untreated and treated groups, respectively, showing that the untreated group weighed significantly less than the treated group (p=0.019). BMI values were 16.92±1.98 kg/m2 and 18.14±2.52 kg/m2 in the untreated and treated groups, respectively; the BMI SDSs were –0.03±0.90 and 0.43±1.05 in the untreated and treated groups, respectively, indicating that patients in the former group had a significantly lower BMI than those in the latter group (p=0.002 and p=0.009, respectively). There were no significant differences in height, height SDS, weight SDS, or MPH between the two groups (p=0.507, p=0.381, p=0.199, and p=0.074, respectively).
The peak serum LH levels after the GnRH stimulation test were significantly different between the two groups (untreated group, 10.31±5.74 IU/L; treated group, 13.36±10.14 IU/L; p=0.012).
The PAH for BA values were estimated to be 156.42±4.43 cm and 154.77±3.72 cm in the untreated and treated groups, respectively, indicating that patients in the untreated group were significantly taller than those in the treated group (p=0.041). The PAH by BP values were estimated to be 157.25±6.25 cm and 155.03±5.71 cm in the untreated and treated groups, respectively, showing no statistically significant difference between the groups (p=0.052).
Table 2 compares the data of the two groups at the time of growth completion. The mean CA of the untreated group at the time of FAH investigation was 15.21±1.89 years.
All patients in the untreated group had menarche at the time of FAH investigation, and the mean onset age of menstruation was 11.37±0.89 years. In the treated group, 63 of the 206 patients had their first menstruation at the time of FAH investigation after the end of treatment, and the mean age of onset was 12.36±0.60 years. The age at the onset of menstruation was significantly earlier in the untreated group than in the treated group (p<0.001). The FAHs were 160.71±4.56 cm and 159.31±4.26 cm in the untreated and treated groups, respectively, with no significant difference between them (p=0.114).
The FAH in the untreated group was greater than the PAH for BA and PAH by BP, but similar to the MPH (Fig. 1). The FAH was 4.29±3.33 cm greater than PAH for BA, and the difference was statistically significant (p<0.001). The FAH was significantly larger than PAH by BP by 3.46±3.93 cm (p<0.05). Although the FAH was 0.99±4.50 cm shorter than the MPH, the difference was not statistically significant (p=0.4).
The FAH in the treated group was similar to the MPH, but the difference was not significant (p=0.057). The FAH was significantly greater than PAH for BA and PAH by BP by 4.54±0.50 cm and 4.29±0.58 cm (p<0.001 and p<0.001), respectively.
This study compared PAH using the height SDSs for BA and PAH using the BP method at diagnosis and height at the completion of growth in girls diagnosed with CPP without treatment. In addition, we compared the clinical characteristics of girls with CPP who were and were not treated with GnRH agonists at 8 to 9 years of age. We found that the FAH in the untreated group was significantly higher than the predicted height at the time of CPP diagnosis, and the onset age of menstruation was younger in the untreated group than in the treated group.
The number of patients diagnosed with precocious puberty in Korea is rapidly increasing. According to the 2019 Korea National Health and Nutrition Examination Survey released by the Health Insurance Review and Assessment Service, the incidence of CPP in girls increased 4.6-fold between 2008 and 2014, from 89.4 per 100,000 people to 415.3 per 100,000 people. The most common ages at diagnosis were 8 and 9 years, with an incidence of 1642.7 per 100,000 people, followed by 7 and 8 years, with an incidence of 440.6 per 100,000 people [9]. Girls in whom breast development is observed have significantly more advanced BA than CA, and those with an adolescent response to the GnRH test are treated with GnRH agonists to inhibit pubertal progression and improve FAH [10].
The course of precocious puberty varies, ranging from rapidly progressive manifestations to slowly progressing puberty or completely regressing puberty symptoms that require no treatment [11,12]. Fontoura et al. [13] described a form of precocious puberty in which pubertal development starts early but progresses slowly. The pathophysiological mechanism of slowly progressive precocious puberty is not yet known [14], and preventing unnecessary treatment by identifying the transient or slowly progressive form is crucial [15].
GnRH agonist treatment seems to improve adult height, particularly in girls diagnosed with CPP before 6 years of age [16], but its actual benefit in terms of adult height for patients who begin puberty after 6 years of age remains controversial. Léger et al. [7] stated that medical treatment is unnecessary for all patients because pubertal responsiveness to GnRH, apparent impairment of height potential, and significantly advanced BA do not always persist in patients with idiopathic CPP. Guaraldi et al. [17] reported no height benefit in patients treated with a GnRH agonist after 8 years of age. Kreiter et al. [18] reported an improvement in PAH in seven girls with CPP after 2 years of follow-up without treatment. Cassio et al. [19] reported that the final height did not differ between GnRH agonist-treated and untreated groups. Brauner et al. [20] reported that growth potential was preserved in 15 girls with tall PAH at initial assessment and slow puberty; therefore, the FAH was similar to the target height in this group.
PAH in children is estimated using the BP method [21] or the Tanner-Whitehouse method [22], using CA, BA, and height. As this method was developed for the Caucasian population, it may not be suitable for Koreans. Hence, this study used a method of estimating PAH by first calculating the height SDS corresponding to BA at the time of diagnosis by referring to the 2017 Korean National Growth Charts for children and adolescents [23] and then substituting this height SDS into the female height SDS of an 18-year-old to 11-month-old girl close to adulthood.
We also found that girls diagnosed with CPP between 8 and 9 years of age grew close to the MPH and had a taller PAH than that at the time of CPP diagnosis, even without treatment with GnRH agonists. However, a taller-than-average height SDS, smaller BA-CA gap, and an expected height that was not remarkably short in the untreated patients seemed to have influenced the parents’ decision not to proceed with GnRH agonist treatment.
This study had several limitations. The main limitation was that most patients diagnosed with CPP initiated treatment with a GnRH agonist; therefore, the sample size of the untreated patients was inevitably small. In addition, since information on FAH and the onset of menstruation was obtained through a survey administered to the patients’ parents, there may have been recollection errors.
In conclusion, this study found that the FAH was greater than the PAH at diagnosis in children diagnosed with CPP between 8 and 9 years of age without treatment and that they grew close to the MPH. Furthermore, when pubertal onset is late, the initial Tanner stage is low, or the peak LH level is low, even if CPP is diagnosed, patient observation without treatment may be possible.
Therefore, for girls diagnosed with CPP, it is recommended to consider various conditions, such as pubertal onset, height at diagnosis, BA, peak LH level, predicted height, and speed of puberty, when deciding whether to administer GnRH agonists.
References
1. Bajpai A, Menon PS. Contemporary issues in precocious puberty. Indian J Endocrinol Metab. 2011; 15(Suppl 3):S172–9.

2. Kim JH, Shin CH, Lee SY. Observed trends for an earlier onset of puberty: when is the need for treatment indicated? J Korean Med Assoc. 2009; 52:1189–200.

3. Bertelloni S, Baroncelli GI, Sorrentino MC, Perri G, Saggese G. Effect of central precocious puberty and gonadotropin-releasing hormone analogue treatment on peak bone mass and final height in females. Eur J Pediatr. 1998; 157:363–7.

4. Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab. 2013; 98:2198–207.

5. Lahlou N, Carel JC, Chaussain JL, Roger M. Pharmacokinetics and pharmacodynamics of GnRH agonists: clinical implications in pediatrics. J Pediatr Endocrinol Metab. 2000; 13(Suppl 1):723–37.

6. Allali S, Lemaire P, Couto-Silva AC, Prété G, Trivin C, Brauner R. Predicting the adult height of girls with central precocious puberty. Med Sci Monit. 2011; 17:PH41–8.

7. Léger J, Reynaud R, Czernichow P. Do all girls with apparent idiopathic precocious puberty require gonadotropin-releasing hormone agonist treatment? J Pediatr. 2000; 137:819–25.

8. Bouvattier C, Coste J, Rodrigue D, Teinturier C, Carel JC, Chaussain JL, et al. Lack of effect of GnRH agonists on final height in girls with advanced puberty: a randomized long-term pilot study. J Clin Endocrinol Metab. 1999; 84:3575–8.

9. Kim YJ, Kwon A, Jung MK, Kim KE, Suh J, Chae HW, et al. Incidence and prevalence of central precocious puberty in Korea: an epidemiologic study based on a national database. J Pediatr. 2019; 208:221–8.

10. Allen NG, Krishna KB, Lee PA. Use of gonadotropin-releasing hormone analogs in children. Curr Opin Pediatr. 2021; 33:442–8.

11. Carel JC, Léger J. Clinical practice: precocious puberty. N Engl J Med. 2008; 358:2366–77.
12. Lanes R, Soros A, Jakubowicz S. Accelerated versus slowly progressive forms of puberty in girls with precocious and early puberty: gonadotropin suppressive effect and final height obtained with two different analogs. J Pediatr Endocrinol Metab. 2004; 17:759–66.

13. Fontoura M, Brauner R, Prevot C, Rappaport R. Precocious puberty in girls: early diagnosis of a slowly progressing variant. Arch Dis Child. 1989; 64:1170–6.

14. Klein KO. Precocious puberty: who has it?: who should be treated? J Clin Endocrinol Metab. 1999; 84:411–4.

15. Palmert MR, Malin HV, Boepple PA. Unsustained or slowly progressive puberty in young girls: initial presentation and long-term follow-up of 20 untreated patients. J Clin Endocrinol Metab. 1999; 84:415–23.

16. Lazar L, Padoa A, Phillip M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J Clin Endocrinol Metab. 2007; 92:3483–9.

17. Guaraldi F, Beccuti G, Gori D, Ghizzoni L. Management of endocrine disease: long-term outcomes of the treatment of central precocious puberty. Eur J Endocrinol. 2016; 174:R79–87.

18. Kreiter M, Burstein S, Rosenfield RL, Moll GW Jr, Cara JF, Yousefzadeh DK, et al. Preserving adult height potential in girls with idiopathic true precocious puberty. J Pediatr. 1990; 117:364–70.

19. Cassio A, Cacciari E, Balsamo A, Bal M, Tassinari D. Randomised trial of LHRH analogue treatment on final height in girls with onset of puberty aged 7.5-8.5 years. Arch Dis Child. 1999; 81:329–32.

20. Brauner R, Adan L, Malandry F, Zantleifer D. Adult height in girls with idiopathic true precocious puberty. J Clin Endocrinol Metab. 1994; 79:415–20.

21. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952; 40:423–41.

22. Tanner JM, Healy MJ, Goldstein H, Cameron N. Assessment of skeletal maturity and prediction of adult height (TW3 method). 3rd ed. London: WB Saunders;2001. p. 110.
Fig. 1.
Height differences in the untreated group. Dif., difference; MPH, midparental height; PAH, predicted adult height; BA, bone age; FAH, final adult height; BP, Bayley-Pinneau. *p<0.05, statistically significant differences between the two groups.
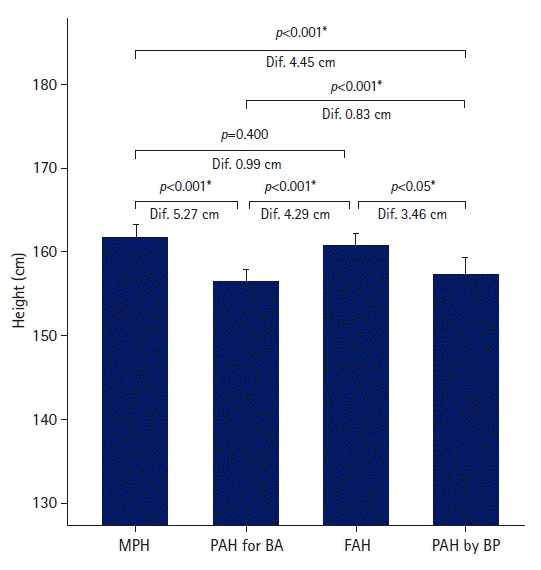
Table 1.
Clinical characteristics of the untreated and treated groups at diagnosis of central precocious puberty
| Characteristic | Untreated group | Treated group | p-value |
|---|---|---|---|
| No. of patients | 36 | 206 | |
| Pubertal onset (yr) | 7.92±0.63 | 7.88±0.32 | 0.674 |
| CA (yr) | 8.34±0.74 | 8.60±0.30 | 0.044* |
| BA (yr) | 9.98±0.89 | 10.44±0.75 | 0.005* |
| BA-CA (yr) | 1.64±0.91 | 1.84±0.67 | 0.116 |
| Tanner stage | 2.08±0.28 | 2.31±0.49 | <0.001* |
| Height (cm) | 133.19±5.95 | 133.90±5.15 | 0.507 |
| Height SDS | 0.81±1.18 | 0.63±0.85 | 0.381 |
| Height SDS for BA | –0.98±0.94 | –1.32±0.79 | 0.043* |
| Weight (kg) | 30.21±5.56 | 32.68±5.91 | 0.019* |
| Weight SDS | 0.38±0.95 | 0.60±0.93 | 0.199 |
| BMI (kg/m2) | 16.92±1.98 | 18.14±2.52 | 0.002* |
| BMI SDS | –0.03±0.90 | 0.43±1.05 | 0.009* |
| LH (IU/L) | 10.31±5.74 | 13.36±10.14 | 0.012* |
| MPH (cm) | 161.69±4.47 | 160.26±3.62 | 0.074 |
| PAH for BA (cm) | 156.42±4.43 | 154.77±3.72 | 0.041* |
| PAH by BP method (cm) | 157.25±6.25 | 155.03±5.71 | 0.052 |
Table 2.
Comparison of characteristics between the untreated and treated groups at the end of growth
| Characteristic | Untreated group (n=36) | Treated group (n=206) | p-value |
|---|---|---|---|
| CA (yr) | 15.21±1.89 | 12.17±0.75 (n=101) | <0.001* |
| Height (cm) | 160.71±4.56 | 159.31±4.26 (n=101) | 0.114 |
| Onset of menstruation (yr) | 11.37±0.89 | 12.36±0.60 (n=63) | <0.001* |
| ΔFAH-MPH (cm) | –0.99±4.50 | –1.03±4.60 | 0.957 |
| ΔFAH-PAH for BA (cm) | 4.29±3.33 | 4.51±2.85 | 0.721 |
| ΔFAH-PAH by BP method (cm) | 3.46±3.93 | 4.17±3.89 | 0.355 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download