Introduction
Case
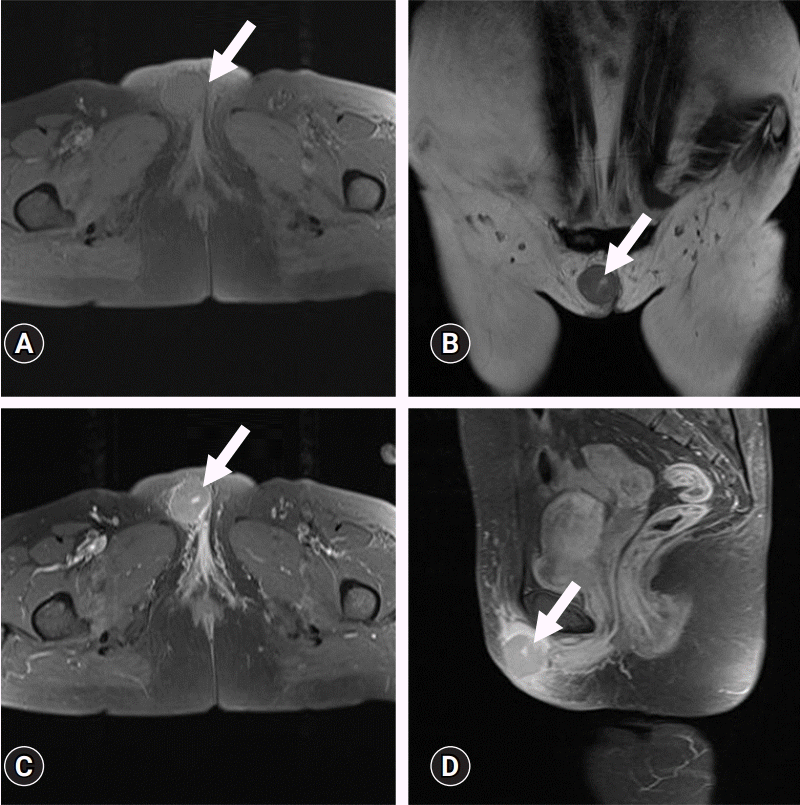 | Fig. 1.Magnetic resonance images show an approximately 3.3 cm, well-defined, round soft tissue mass in the subcutaneous fat layer of the right vulva (arrows). (A) Axial fat-saturated T1-weighted and (B) T2-weighted images show homogeneous iso-signal intensity of the mass with a central focal curvilinear fluid-equivalent signal intensity lesion. (C) Contrast-enhanced axial and (D) sagittal fat-saturated T1-weighted images demonstrate homogeneous, mild enhancement of the mass with surrounding subcutaneous swelling and enhancement. The intralesional focal curvilinear lesion shows strong enhancement. |
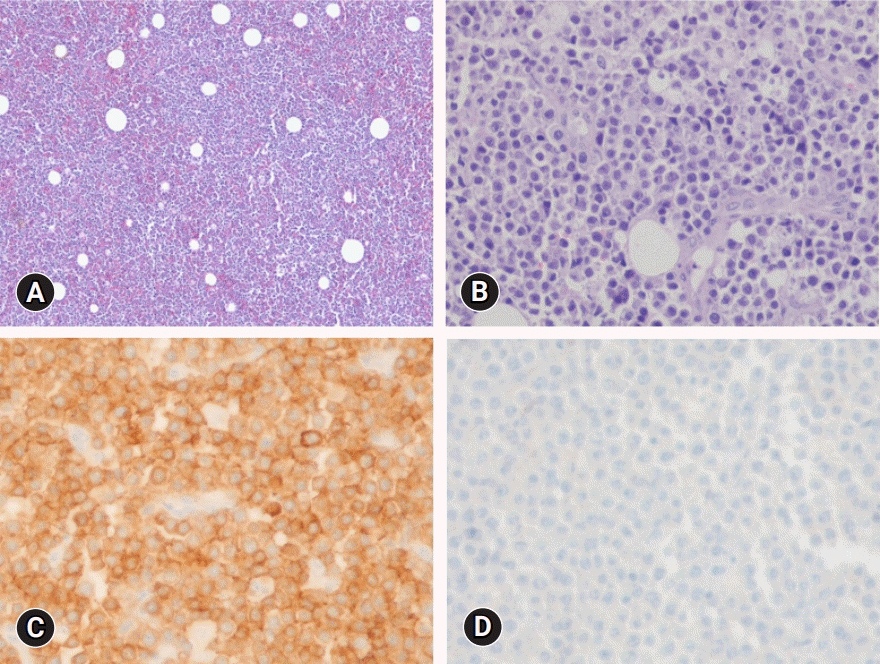 | Fig. 2.Histopathologic findings of the vulva mass. (A) The lymphoid cells are diffusely infiltrated (hematoxylin and eosin stain, x40). (B) Medium-to-large-sized lymphoid cells are present (hematoxylin and eosin stain, x200). (C) The tumor cells are strongly positive for CD79a (immunohistochemical stain, x200). (D) The tumor cells are negative for CD3 (immunohistochemical stain, x200). |
Discussion
Table 1.
| No. | Histopathology | Stagea) | Age (yr) | Size (cm) | First-line treatment | Treatment dateb) | Response to first-line treatment | DFS (mo) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DLBCL (non-GCB type) | IE | 55 | 3.3 | Local excision+6 cycles of R-CHOP+radiation | May 26, 2020 | Complete remission | 29 mo | Current report |
| 2 | PCDLBCL-LT | IE | 38 | 1.0–2.0 | Radiation | NA | Complete remission | 84 yr | Ye et al. 2018 [4] |
| 3 | DLBCL (GCB type) | IE | 73 | 4×2×1.5 | Local excision | NA | Complete remission | 65 mo | Ye et al. 2018 [4] |
| 4 | DLBCL (GCB type) | IIE | 43 | 3.2 | Local excision+6 cycles of R-CHOP | NA | Complete remission | NA | Clemente et al. 2016 [5] |
| 5 | DLBCL | IIEA | 37 | 12×6 | 4 Cycles of R-CHOP+radiation | NA | Complete remission | 36 mo | El Kacemi et al. 2015 [8] |
| 6 | DLBCL | NA | NA | NA | NA | NA | NA | NA | Plaza et al. 2011 [9] |
| 7 | DLBCL | IE | 75 | NA | 5 Cycles of CHOP | NA | Recurrence | 10 mo | Signorelli et al. 2007 [3] |
| 8 | DLBCL | IE | 73 | 3×1.5 | Local excision+radiation | NA | Recurrence | 6 mo | Tjalma et al. 2002 [10] |
| 9 | DLBCL | IIE | 67 | NA | Chemotherapy+radiation | NA | NA | NA | Vang et al. 2001 [11] |
| 10 | DLBCL | IE | 71 | NA | NA | NA | NA | NA | |
| 11 | DLBCL | NA | 68 | NA | NA | NA | NA | NA | |
| 12 | DLBCL | IE | 64 | 3×2 | CHOP | NA | Complete remission | 12 mo | Iczkowski et al. 2000 [12] |
| 13 | DLBCL | IEB | 25 | 4×5.5 | Local excision+CHOP+radiation | Nov 1, 1991 | Recurrence | 6 mo | Kaplan et al. 1996 [13] |
| 14 | DLBCL | IE | 68 | 5×4.5×2.5 | Local excision | Mar, 1991 | NA | 14 mo | Nam et al. 1992 [14] |
DLBCL, diffuse large B-cell lymphoma; DFS, disease-free survival; GCB, germinal center B-cell-like; PCDLBCL-LT, primary cutaneous DLBCL leg type; NA, not applicable; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download