Abstract
The gut microbiota has been reported to exert a significant influence on various physiological responses of hosts. Extensive evidence has recently emerged linking metabolic and cardiovascular disorders to the gut microbiota. Nonalcoholic fatty liver disease (NAFLD) is the most common underlying metabolic disorder, and its prevalence is increasing worldwide. In this study, we aim to review the relationship between the gut microbiota and NAFLD, and explore the potential of the gut microbiota as a novel target for NAFLD treatment.
Go to : 
The gut microbiota in adults is known to comprise between 10 and 100 trillion microorganisms, a quantity that is more than 10-fold the number of human cells [1]. Moreover, the collective genomes of the gut microbiota are 100 to 150 times greater than that of the human genome [2]. Several metagenomic studies have suggested a correlation between the quantity or diversity of genes in the gut microbiota and the health of the host [3-5]. The gut microbiota has evolved alongside human evolution and has been found to significantly influence various physiological responses of the host. Notably, recent studies have demonstrated that alterations in the gut microbial composition are associated with various metabolic diseases, including obesity [6], nonalcoholic fatty liver disease (NAFLD) [7], type 2 diabetes [8], and cardiovascular disease [9-11].
More than one-third of the global population is affected by NAFLD, and the prevalence of this condition has significantly increased [12]. NAFLD induces insulin resistance and generates numerous inflammatory cytokines, bile acid, and cholesterol. Collectively, these factors can lead to type 2 diabetes, and conversely, type 2 diabetes can exacerbate NAFLD [13-17]. Furthermore, NAFLD is a significant contributor to the onset of cardiovascular diseases [18,19]. NAFLD can progress to liver inflammation and hepatocyte damage, resulting in nonalcoholic steatohepatitis (NASH). In some patients, NASH can cause slow, progressive, and severe liver damage, including fibrosis and ultimately, liver cirrhosis (LC). Therefore, NAFLD acts as a fundamental underlying condition contributing to various metabolic disorders.
In this article, we aim to review the relationship between the gut microbiota and NAFLD, as established by various studies to date. We also explore the potential of the gut microbiota as a novel therapeutic target for NAFLD in the future.
Go to : 
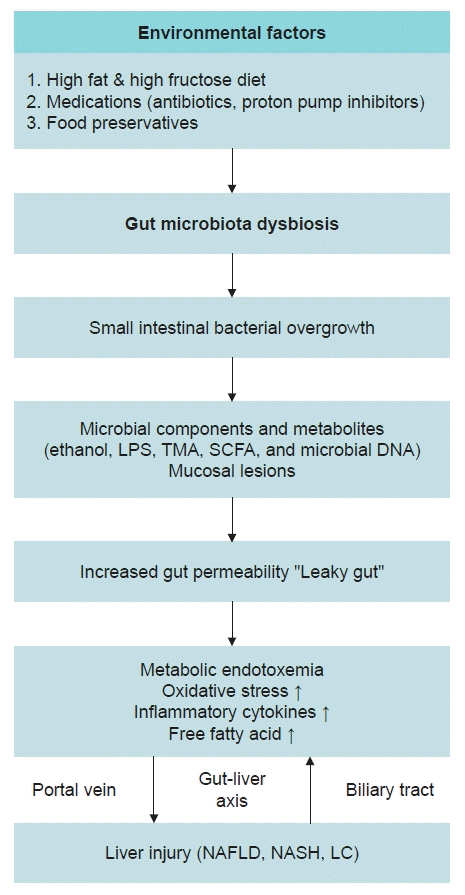
Dysbiosis of the gut microbiota is recognized as a cause of NAFLD and NASH. Various environmental factors influence this dysbiosis. Notably, a diet high in fat and fructose, extensive exposure to medications such as antibiotics and proton pump inhibitors, and various food preservatives are identified as significant environmental contributors to gut microbiota dysbiosis [20-24]. Fructose, which is a monosaccharide naturally found in fruits and honey, has been demonstrated to play a substantial role in the pathogenesis of NAFLD and NASH in both preclinical and clinical studies [25-28], and it is known to induce gut microbiota dysbiosis [29,30]. In addition to its impact on gut microbiota, fructose also has direct, detrimental effects on the liver. Its unique metabolic pathway leads to ATP depletion, uric acid generation, mitochondrial dysfunction, de novo lipogenesis, and the inhibition of beta-fatty oxidation [31-35].
Small internal bacterial overgrowth and gut leakiness play a key role in the occurrence and progression of NAFLD (Fig. 1). Small internal bacterial overgrowth is commonly triggered by an imbalance in gut microbiota [36]. This imbalance leads to an increase in various microbial components and metabolites, including ethanol, lipopolysaccharide, trimethylamine, short-chain fatty acids, and microbial DNA. These elements, along with intestinal mucosal lesions, contribute to increased gut permeability [37,38]. Gut leakiness can also result from dysfunction in the structures of the intestinal barrier. Proteins in the tight junctions serve as crucial mucosal barriers that prevent bacterial translocation. When these proteins are damaged, the translocation of microbial metabolic products, such as lipopolysaccharide, into the bloodstream is increased. This process induces a state of endotoxemia, triggering inflammation in the liver [37,38]. The endotoxemia caused by increased intestinal permeability and the subsequent translocation to the liver are critical factors in the development of NAFLD (Fig. 1).
The gut-liver axis refers to the communication between the gut and the liver. This communication is bidirectional and occurs through the biliary tract, portal vein, and systemic circulation (Fig 1). Endotoxins that reach the liver via the portal vein interact with receptors such as Toll-like receptors 4 or 9 (TLR4 or TLR9). TLR4 is found on the cell membranes of hepatocytes and immune cells, specifically Kupffer cells. TLR4 facilitates the activation of molecules such as NF-κB, which in turn activate inflammatory cytokines [39,40]. The biliary tract plays a pivotal role in enabling bidirectional communication between the liver and the intestine. Substances derived from the liver significantly influence both the composition of the gut microbiota and the integrity of the gut barrier [41]. These processes can aggravate liver damage. NAFLD, NASH, and LC are often viewed as a continuum, with shared pathways influenced by the gut microbiota. However, there is still a substantial gap in research concerning the specific mechanisms by which the gut microbiota uniquely contributes to the progression from NAFLD to NASH and LC. Future research should aim to uncover these unique mechanisms for each condition and gain a better understanding of how the gut microbiota may impact these processes. In conclusion, maintaining the integrity of tight junctions and inhibiting gut microbiota dysbiosis could be an effective strategy for preventing or treating NAFLD and other gut-related diseases.
Go to : 
Patients with NAFLD have been found to exhibit alterations in their gut microbiota compared to healthy individuals. Notably, the gut microbiota signatures associated with NAFLD include an increase in the Proteobacteria phylum, the Enterobacteriaceae family, and the Escherichia, Bacteroides, Dorea, and Peptoniphilus genera. Conversely, there is a decrease in the Rikenellaceae and Ruminococcaceae families, and the Faecalibacterium, Coprococcus, Anaerosporobacter, and Eubacterium genera [42-46]. However, interventional clinical studies to determine whether these specific species cause NAFLD, in order to establish causality, are not feasible due to observations from several studies that these species change following bariatric metabolic surgery [47-49].
Certain specific species have been utilized in the treatment of NAFLD, and the results have shown promise in improving the condition [50,51]. The most used probiotics belong to the Lactobacillus genus, as follows: Lactobacillus casei, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus bulgaricus, and Lactobacillus acidophilus [52-57]. Others include Bifidobacteria and Streptococcus thermophiles [58]. Numerous studies have recently explored the combination of multiple species of probiotics [59-61].
Go to : 
Numerous studies have targeted the gut microbiota for therapeutic and preventative interventions. These interventions encompass probiotics, prebiotics, synbiotic supplements, and fecal microbiota transplantation (FMT). In the context of NAFLD treatment, research has been conducted to alter the gut microbiota composition and reestablish balance through the administration of probiotics, prebiotics, and synbiotic supplements. Probiotics are specific species that could offer beneficial effects. Prebiotics have recently been defined as indigestible dietary components that selectively stimulate the growth and activity of beneficial gut bacteria. This definition has been broadened to include not only indigestible carbohydrates such as fructooligosaccharides, galactooligosaccharides, and trans-galactooligosaccharides, but also other substances like polyunsaturated fatty acids and polyphenols that can modulate the gut microbiota [62,63]. Synbiotics are defined as a mixture of probiotics and prebiotics.
Animal studies have shown that probiotics can slow the progression of NAFLD [64,65]. Furthermore, a meta-analysis of clinical studies, in which patients with NAFLD were treated with probiotics, revealed significant reductions in alanine aminotransferase, aspartate aminotransferase, and total cholesterol within the probiotics group [66,67]. Although the number of patients included in these studies is limited, making it challenging to evaluate any actual changes in the composition of the intestinal microflora posttreatment, probiotics, prebiotics, and synbiotic supplements are associated with minimal side effects. Therefore, the results of future research are eagerly anticipated. A randomized controlled study of FMT, where fecal bacteria from healthy individuals are transplanted into NAFLD patients, has also been recently published [68]. The group that underwent FMT showed improved intestinal permeability. However, there was no observed difference in insulin resistance or intrahepatic fat [68]. In the same study, while an increase in bacterial diversity was noted, there were no definitive changes in the composition of the microbiota. This lack of change in microbiota composition may be due to the administration of FMT into the duodenum. Stool specimen analysis may not accurately reflect changes in the microbiome of the small intestine or the proximal colon. Conversely, another clinical trial where FMT was administered via colonoscopy demonstrated changes in both the composition of the microbiota and fatty liver post-FMT [69]. Therefore, further research is needed to explore the therapeutic effects of FMT in patients with NAFLD.
Another promising area for therapeutic intervention lies in factors associated with bile acid metabolism. Bile acids serve to prevent intestinal bacterial overgrowth, both directly and indirectly. Obeticholic acid (OCA), a potent activator of the farnesoid X receptor, has been shown to improve hepatic steatosis and fibrosis in animal studies [70]. Furthermore, OCA has been found to reduce bacterial translocation and improve gut microbiota dysbiosis in rats with LC [71]. A phase 3 clinical trial with OCA demonstrated a protective effect against fibrosis, as confirmed by biopsy [72]. However, despite these promising results, numerous patients have reported unusual observations, such as dermatological manifestations, during clinical trials. Consequently, the applicability of OCA to patients remains unconfirmed and is a subject of ongoing debate. Fibroblast growth factor 19 is a gut hormone that plays a major role in regulating bile acid metabolism [73,74]. The fibroblast growth factor 19 analog NGM282, which regulates bile acid synthesis and glucose homeostasis, has been shown to reduce hepatic steatosis in patients with NASH. A phase 2 study of NAFLD with NGM282, published in 2018, revealed that the treatment group experienced significant reductions in intrahepatic fat, fibrosis-related markers, and intrahepatic fat content within 12 weeks [75]. Regarding drug side effects, only mild symptoms such as digestive discomfort and pain at the injection site have been reported [75]. Further research results on the use of NGM282 to treat NAFLD and NASH are expected in the future.
Go to : 
Gut microbiota dysbiosis resulting from various environmental factors causes NAFLD. There have been attempts to identify treatment targets for NAFLD through studies on the mechanisms through which gut dysbiosis causes NAFLD. Moreover, numerous studies have shown improvements in NAFLD by directly restoring the composition of intestinal microbiota through probiotics, prebiotics, synbiotics, and FMT. In the future, studies exploring how the gut microbiome could be targeted for the treatment of NAFLD are expected.
Go to : 
Notes
Author contributions
Conceptualization: Boyeon Kim. Writing - original draft: Boyeon Kim. Writing - review & editing: Bukyung Kim. Approval of final manuscript: Boyeon Kim, Bukyung Kim.
Go to : 
References
1. Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010; 28:623–67.

2. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010; 464:59–65.

3. Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013; 500:585–8.

4. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013; 500:541–6.

5. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009; 457:480–4.

6. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444:1027–31.

7. Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Transl Res. 2017; 179:49–59.

8. Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013; 498:99–103.

9. Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016; 12:169–81.

10. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013; 368:1575–84.

11. Tang WH, Hazen SL. The gut microbiome and its role in cardiovascular diseases. Circulation. 2017; 135:1008–10.

12. Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022; 7:851–61.

13. Rhee EJ. Nonalcoholic fatty liver disease and diabetes: an epidemiological perspective. Endocrinol Metab (Seoul). 2019; 34:226–33.

14. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017; 14:32–42.

15. Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. 2019; 40:1367–93.

16. Simonen P, Kotronen A, Hallikainen M, Sevastianova K, Makkonen J, Hakkarainen A, et al. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. J Hepatol. 2011; 54:153–9.

17. Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009; 104:861–7.

18. Caussy C, Aubin A, Loomba R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr Diab Rep. 2021; 21:15.

19. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013; 10:330–44.

20. Hrncir T, Hrncirova L, Kverka M, Hromadka R, Machova V, Trckova E, et al. Gut microbiota and NAFLD: pathogenetic mechanisms, microbiota signatures, and therapeutic interventions. Microorganisms. 2021; 9:957.

21. Hrncirova L, Machova V, Trckova E, Krejsek J, Hrncir T. Food preservatives induce Proteobacteria dysbiosis in human-microbiota associated Nod2-deficient mice. Microorganisms. 2019; 7:383.

22. Hrncirova L, Hudcovic T, Sukova E, Machova V, Trckova E, Krejsek J, et al. Human gut microbes are susceptible to antimicrobial food additives in vitro. Folia Microbiol (Praha). 2019; 64:497–508.

23. Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017; 66:1414–27.

24. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014; 514:181–6.

25. Mock K, Lateef S, Benedito VA, Tou JC. High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J Nutr Biochem. 2017; 39:32–9.

26. Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018; 68:1063–75.

27. Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014; 100:833–49.
28. Sobrecases H, Le KA, Bortolotti M, Schneiter P, Ith M, Kreis R, et al. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab. 2010; 36:244–6.

29. Moughaizel M, Dagher E, Jablaoui A, Thorin C, Rhimi M, Desfontis JC, et al. Long-term high-fructose high-fat diet feeding elicits insulin resistance, exacerbates dyslipidemia and induces gut microbiota dysbiosis in WHHL rabbits. PLoS One. 2022; 17:e0264215.

30. Li JM, Yu R, Zhang LP, Wen SY, Wang SJ, Zhang XY, et al. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome. 2019; 7:98.

31. Maenpaa PH, Raivio KO, Kekomaki MP. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968; 161:1253–4.

32. Bawden SJ, Stephenson MC, Ciampi E, Hunter K, Marciani L, Macdonald IA, et al. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: a (31)P MRS study. Clin Nutr. 2016; 35:645–9.

33. Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986; 21:1–32.
34. Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012; 287:40732–44.
35. Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012; 7:e48801.

36. Rao SS, Bhagatwala J. Small intestinal bacterial overgrowth: clinical features and therapeutic management. Clin Transl Gastroenterol. 2019; 10:e00078.

37. Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015; 5:8096.

38. Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, et al. Loss of junctional adhesion molecule A promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology. 2016; 151:733–46.

39. Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013; 28(Suppl 1):38–42.

40. Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, et al. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015; 309:G270–8.

41. Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018; 15:397–411.

42. Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017; 16:375–81.

43. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013; 57:601–9.

44. Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017; 25:1054–62.

45. Hoyles L, Fernandez-Real JM, Federici M, Serino M, Abbott J, Charpentier J, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018; 24:1070–80.

46. Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013; 11:868–75.

47. Hoozemans J, de Brauw M, Nieuwdorp M, Gerdes V. Gut microbiome and metabolites in patients with NAFLD and after bariatric surgery: a comprehensive review. Metabolites. 2021; 11:353.

48. Xia Y, Ren M, Yang J, Cai C, Cheng W, Zhou X, et al. Gut microbiome and microbial metabolites in NAFLD and after bariatric surgery: correlation and causality. Front Microbiol. 2022; 13:1003755.

49. Kim Y, Son D, Kim BK, Kim KH, Seo KW, Jung K, et al. Association between the Blautia/Bacteroides ratio and altered body mass index after bariatric surgery. Endocrinol Metab (Seoul). 2022; 37:475–86.

50. Chen J, Vitetta L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int J Mol Sci. 2020; 21:5214.

51. Briskey D, Heritage M, Jaskowski LA, Peake J, Gobe G, Subramaniam VN, et al. Probiotics modify tight-junction proteins in an animal model of nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2016; 9:463–72.

52. Wagnerberger S, Spruss A, Kanuri G, Stahl C, Schroder M, Vetter W, et al. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. J Nutr Biochem. 2013; 24:531–8.

53. Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, et al. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011; 110:650–7.

54. Zhao Z, Chen L, Zhao Y, Wang C, Duan C, Yang G, et al. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol. 2020; 104:5273–82.

55. Park EJ, Lee YS, Kim SM, Park GS, Lee YH, Jeong DY, et al. Beneficial effects of Lactobacillus plantarum strains on non-alcoholic fatty liver disease in high fat/high fructose diet-fed rats. Nutrients. 2020; 12:542.

56. Kim B, Park KY, Ji Y, Park S, Holzapfel W, Hyun CK. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2016; 473:530–6.

57. Anderson JW, Gilliland SE. Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J Am Coll Nutr. 1999; 18:43–50.

58. Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011; 15:1090–5.
59. Ferolla SM, Armiliato GN, Couto CA, Ferrari TC. Probiotics as a complementary therapeutic approach in nonalcoholic fatty liver disease. World J Hepatol. 2015; 7:559–65.

60. Arellano-Garcia L, Portillo MP, Martinez JA, Milton-Laskibar I. Usefulness of probiotics in the management of NAFLD: evidence and involved mechanisms of action from preclinical and human models. Int J Mol Sci. 2022; 23:3167.

61. Lavekar AS, Raje DV, Manohar T, Lavekar AA. Role of probiotics in the treatment of nonalcoholic fatty liver disease: a meta-analysis. Euroasian J Hepatogastroenterol. 2017; 7:130–7.

62. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995; 125:1401–12.

63. Vamanu E. Complementary functional strategy for modulation of human gut microbiota. Curr Pharm Des. 2018; 24:4144–9.

64. Zhao Z, Wang C, Zhang L, Zhao Y, Duan C, Zhang X, et al. Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl Microbiol Biotechnol. 2019; 103:5843–50.

65. Kim DH, Kim H, Jeong D, Kang IB, Chon JW, Kim HS, et al. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: targeted and untargeted community analysis with correlation of biomarkers. J Nutr Biochem. 2017; 44:35–43.

66. Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013; 19:6911–8.

67. Zhou X, Wang J, Zhou S, Liao J, Ye Z, Mao L. Efficacy of probiotics on nonalcoholic fatty liver disease: a meta-analysis. Medicine (Baltimore). 2023; 102:e32734.

68. Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol. 2020; 115:1055–65.

69. Xue L, Deng Z, Luo W, He X, Chen Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front Cell Infect Microbiol. 2022; 12:759306.

70. An P, Wei G, Huang P, Li W, Qi X, Lin Y, et al. A novel non-bile acid FXR agonist EDP-305 potently suppresses liver injury and fibrosis without worsening of ductular reaction. Liver Int. 2020; 40:1655–69.

71. Ubeda M, Lario M, Munoz L, Borrero MJ, Rodriguez-Serrano M, Sanchez-Diaz AM, et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol. 2016; 64:1049–57.

72. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019; 394:2184–96.
73. Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015; 33:327–31.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download