See letter "Omega-3 fatty acids: promising therapeutic agents for combating kidney injuries" in Volume 38 on page 157.
Abstract
Background
Cyclosporine A (CsA)-induced kidney injury is characterized by renal impairment with inflammatory cell infiltrations, apoptosis, fibrosis, and hypoxic injury. It is not clear whether omega-3 fatty acids (O-3 FAs), which have anti-inflammatory and antioxidant roles, affect nuclear factor erythroid 2-related factor 2 (Nrf2) expression. The aim of this study was to investigate whether O-3 FAs affect Nrf2 expression and exert anti-inflammatory, anti-apoptotic, and anti-fibrotic effects in CsA-induced nephropathy.
Methods
Male Sprague-Dawley rats were divided into control, CsA-treated, and CsA-treated with O-3 FA groups. Nrf2 expression was measured by Western blots and immunohistochemical staining.
Results
Kidney function was impaired in the CsA-treated rats compared to the controls. Caspase-3 and caspase-7 were activated in the CsA-treated group, and the Bax/Bcl2 ratio was higher. O-3 FAs attenuated these apoptosis-related changes. ED-1 and inhibition of kappa B (IĸB) protein expression were significantly upregulated in the CsA-treated group. Compared to the control group, O-3 FA supplementation attenuated the increased expression of ED-1 and IĸB related to inflammation. Smad2/3, Smad4, and transforming growth factor-β1 were activated in the CsA group, and O-3 FA treatment prevented these changes related to renal fibrosis. The expression of Nrf2 was reduced in CsA-treated rats, but Nrf-2 was increased by O-3 FA treatment.
Cyclosporine A (CsA), a calcineurin inhibitor, is an important immunosuppressant used in organ transplantation and autoimmune diseases. It is clinically limited by its nephrotoxicity, which is characterized by a decreased glomerular filtration rate, inducing progressive irreversible renal structural damage, tubulointerstitial inflammation, and striped fibrosis [1]. Recent studies have revealed roles for CsA-induced oxidative stress and apoptosis in causing kidney damage [2,3]. Therefore, it is important to prevent acute CsA nephropathy and retard the progression of chronic CsA nephropathy.
Nuclear factor erythroid 2-related factor 2 (Nrf2) serves as a key transcriptional regulator of the basal and inducible expression of many genes encoding detoxification enzymes, antioxidant proteins, and other stress-response mediators [4]. Previous studies have reported the role of Nrf2 in modulating the expression of cytoprotective genes in the kidney through in vivo and in vitro studies [5,6]. A loss of Nrf2 activity may exacerbate oxidative and inflammatory damage, which is linked to oxidative stress and inflammatory diseases, such as atherosclerosis, diabetes, and rheumatoid arthritis [7-10].
Omega-3 fatty acids (O-3 FAs) comprise a family of polyunsaturated FAs that consist of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). DHA and/or EPA have been reported to reduce plasma triglyceride (TG) levels [11] and improve vascular compliance and vasodilatation to stabilize atherosclerotic plaques [12,13] and cardiovascular events [14,15]. A randomized, double-blind study of dietary fish oil supplementation after renal transplantation reported a beneficial effect on renal hemodynamics and blood pressure and significantly fewer rejection episodes in the fish oil group than in the control group [16]. Furthermore, there was a trend toward increased graft survival [16]. Sakai et al. [17] reported that EPA and DHA have upregulated the Nrf2-mediated antioxidant response, attenuating DNA damage induced by oxidative stress in vascular endothelial cells. Only a few studies have reported that O-3 FA affected Nrf-2 expression [17-20]. In this study, we investigate whether O-3 FA has anti-inflammatory, anti-apoptotic, and anti-fibrotic activities in a rat model of CsA-induced nephropathy. In addition, we evaluated the impact of O-3 FA on the Nrf2 expression in this model.
Ethical statements: The animal procedures were authorized by the Institutional Animal Care Committee of Dong-A University (DIACUC-14-29).
This study included 18 adult male Sprague-Dawley rats weighing 250 to 280 g. The rats were kept in cages that controlled temperature and light. They were fed a low-salt diet (0.05% sodium; Teklad Premier) and tap water.
Rats were divided randomly into three groups. The control group (n=6) was administered saline (1 mL/kg/day) via subcutaneous injection and saline (1 mL/kg/day) by gastric gavage for 4 weeks, the CsA-treated group (n=6) was administered CsA (15 mg/kg/day) via subcutaneous injection and saline (1 mL/kg/day) by gastric gavage for 4 weeks, and the CsA+O-3 FA group (n=6) was administered CsA via subcutaneous injection and O-3 FA (300 mg/kg/day) via gastric gavage for 4 weeks. CsA (Chong Kun Dang Pharm.) was dissolved to a concentration of 15 mg/mL in normal saline, and the dose and administration route were determined based on a previous study of chronic CsA nephropathy [21]. O-3 FA was administered via a gastight micro-syringe (Gastight; Hamilton) and its dose was determined in a previous study [22]. Rats fed in pairs and monitored their daily body weight.
Blood samples were taken from the heart on the day of euthanasia. Whole blood CsA levels were gauged by a monoclonal radioimmunoassay (Incstar Corp.). Blood urea nitrogen and serum creatinine levels were quantified via an automatic analyzer (Roche), and total cholesterol and TG levels were estimated using a colorimetric test kit (Asan Pharmaceutical Co.).
Rat kidney tissues were cleansed with heparinized saline and fixed in a periodate-lysine-paraformaldehyde solution, and wax-embedded kidney tissues were cut into 4 μm sections. Kidney sections were stained with periodic acid-Schiff. The kidney lesions, including vacuolization of the tubular cells, tubular atrophy and dilatation, and inflammatory cellular infiltration and interstitial fibrosis, were viewed by an Aperio ScanScope (Aperio Technologies).
Proteins were extracted from kidney tissues using lysis buffer (pH 7.6, including 300 mM NaCl, 50 mM Tris-Cl, 0.5% Triton X-100, and protease inhibitor cocktail). The protein concentrations of the lysates were determined using a Bradford protein assay kit (Bio-Rad). Protein lysates were separated using 7.5% to 15% SDS/PAGE gels and then transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech). After blocking with 1% skim milk, membranes were incubated with primary antibodies overnight at 4 ˚C. Antibodies against transforming growth factor-β1 (TGF-β1), Smad2/3, Smad4, Bcl-2, Bax, and inhibitor of kappa B (IĸB) were acquired from Santa Cruz Biotechnology. Rabbit polyclonal anti-caspase 3 and caspase 7 antibodies were supplied from Cell Signaling Technology. Antibodies against Nrf2 were supplied from Abcam. Antibodies against ED-1 and β-actin were supplied from Serotec and Sigma-Aldrich. Membranes were sequentially incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Immunostaining with antibodies was carried out using the Super Signal West Pico (Thermo Fisher Scientific) enhanced chemiluminescence substance and founded with LAS-3000 Plus (Fuji Photo Film). The response was quantified and regularized to the β-actin control band using ImageJ version 1.48q.
To perform immunohistochemical staining for Nrf2, the kidney sections were transferred into a Tris-EDTA buffer solution (pH 9.0; DAKO). Slides were microwaved at medium power for 7 minutes to retrieve the antigens. To block the endogenous peroxidase activity, 0.3% H2O2 in distilled water was applied to the tissue sections for 20 minutes and incubated for 30 minutes at 37 °C with 5% normal goat serum. The slides were incubated overnight at 4 °C with anti-Nrf2 antibody and additionally incubated with secondary antibody for 1 hour at 37 °C. The slides were then incubated in 3,3-diaminobenzidine with H2O2 substrate and hematoxylin. The negative control group was stained with a buffer solution instead of the primary antibody under the same circumstances. Results were verified by means of an Aperio ScanScope slide scanner.
Results obtained at week 4 are summarized in Table 1. The body weights of each group were at the start of the study. The final body weights of the rats in the CsA-treated and CsA+O-3 FA-treated groups were significantly decreased compared to the control group. At week 4, the serum creatinine and blood urea nitrogen were increased in the CsA-treated group compared to the control group. The CsA+O-3 FA-treated group showed serum creatinine levels similar to those of the CsA-treated group. The total cholesterol and TG levels were also meaningfully raised in the CsA-treated group compared to the control group, but there was no discernible difference between the two groups. The serum cyclosporine levels in the CsA and CsA+O-3 FA groups were over 2,000 ng/mL.
Compared to the control group, the CsA-treated group revealed vacuolization of tubular cells, tubular atrophy, and interstitial fibrosis in the periodic acid-Schiff-stained specimens (Fig. 1). The rats given O-3 FA supplementation had fewer tubulointerstitial alterations than the group treated with CsA alone.
Caspase-3 and caspase-7 expression were activated in the CsA nephropathy, and O-3 FA supplementation attenuated the activation of these effector caspases in CsA nephropathy (Fig. 2). The ratio of Bax and Bcl-2 was higher in the CsA group and was attenuated by O-3 FA in the CsA+O-3 FA-treated group (Fig. 3).
ED-1 and IĸB protein expression were activated in the CsA-treated group compared to the control group. O-3 FA supplementation attenuated the increased ED-1 and IĸB expression (Fig. 4). The total Smad2/3 and Smad4 levels were activated in the CsA-treated group and O-3 FA supplementation attenuated the upregulation of these Smads. An important pro-fibrotic molecule, TGF-β1, was activated in the CsA-treated rats and TGF-β1 was attenuated by O-3 FA supplementation (Fig. 5).
The CsA-treated rats showed significant downregulation of Nrf2. O-3 FA supplementation increased Nrf2 expression compared to CsA treatment (Fig. 6A). Nrf2 expression in the kidney was also confirmed by immunohistochemical staining. Nrf2 expression was mostly manifested in the kidney tubules of the normal rats (Fig. 6B). This was reduced in the CsA-treated rats (Fig. 6C) and restored by O-3 FA supplementation (Fig. 6D).
In this study, we found that O-3 FA upregulates Nrf-2 expression and potentiates anti-inflammatory, anti-apoptotic, and anti-fibrotic processes in CsA-induced nephropathy model. Nrf2 acts as a master regulator of anti-inflammatory and antioxidant gene expression [4]. Previous studies have shown that Nrf2-deficiency enhanced the susceptibility to both ischemic and nephrotoxic renal injury. Compared to wild-type mice, the kidney damage and interstitial fibrosis resulting from CsA treatment were relatively higher in Nrf2-knockout mice [18]. Thus, a potential therapeutic target against kidney injury may be increasing Nrf2 expression and O-3 FA may be a new therapeutic agent for the prevention of CsA-induced kidney injury.
Bardoxolone methyl is an Nrf2 activator, the first orally available antioxidant. In a 52-week bardoxolone methyl treatment of patients with type 2 diabetes mellitus and chronic kidney disease, bardoxolone methyl reduced serum creatinine concentrations [23]. However, bardoxolone methyl did not decrease the risk of end-stage renal disease or death from cardiovascular disease. In addition, heart failure, nonlethal myocardial infarction, nonlethal stroke, hospital admission for heart failure, or death from cardiovascular causes was higher in the bardoxolone methyl group than in the placebo group [24]. In contrast, O-3 FA is beneficial for cardiovascular health and is a natural Nrf2 activator. Further human and animal studies are needed to investigate the effects of O-3 FA on Nrf2. In this study, we also demonstrated Nrf2 expression in the tubular cells of the kidney. Therefore, we suspect that Nrf2, rather than podocytes, may be the predominant protection mechanism against tubular cell injury [25].
Bcl-2 family proteins are the regulators of apoptosis, and the overexpression of Bcl-2 improves cell survival by inhibiting apoptosis [26]. Bax belongs to a family of proteins that share homology with Bcl-2 and the formation of heterodimers between Bax and Bcl-2 homologs with death repressor function leads to suppression of the death-promoting effects of Bax. The relative ratio of Bax and Bcl-2 has been suggested to determine cell survival after apoptotic stimuli [27]. In this study, the relative ratio of Bax and Bcl-2 was changed by O-3 FA, suggesting that O-3 FA prevented apoptosis in the CsA-treated rat model. We also demonstrated that apoptosis-related effector caspase-3 and caspase-7 were inhibited by O-3 FA supplementation in CsA-induced nephropathy.
Nuclear factor-κB (NF-κB) signaling plays an important role in interstitial inflammation in the kidney [28]. In the unstimulated state, NF-κB is present in the cytoplasm attached to the suppressor protein inhibitor of NF-κB, but certain stimulators generate free NF-κB through the degradation and phosphorylation of IκB. Dissociated NF-κB proteins translocate to the nucleus and promote macrophage infiltration and interstitial inflammation [28]. We also observed increased inflammatory processes by increased IκB and pan-macrophage marker ED-1 expression in CsA nephropathy and O-3 FA supplementation attenuated the inflammatory process. In a rat model of progressive proteinuric renal disease, interstitial macrophages produced TGF-β1 and the levels correlated with interstitial inflammatory infiltration [29]. TGF-β1 is a well-known key mediator, activating its downstream Smad signaling pathway in renal fibrosis [30]. In this study, we found that TGF-β1 and a Smad-dependent pathway were inhibited by O-3 FA supplementation.
In conclusion, O-3 FA attenuates tubulointerstitial injury in a CsA-induced nephropathy rat model by mitigating apoptosis, inflammation, and fibrosis and increasing Nrf2 expression. These observations showed the potential role of O-3FA supplementation as an adjunct in the management of delaying CsA-induced nephropathy.
Notes
Author contributions
Conceptualization: WSA. Data curation: JYL, YKS, MHL, SML, WSA. Methodology: JYL, YKS, MHL, SML, SEK, WSA. Formal analysis: MHL, SML, WSA. Supervision: JYL, YKS, MHL, SML, SEK, WSA. Writing - original draft: JYL, YKS, WSA. Writing - review & editing: JYL, YKS, WSA. Approval of final manuscript: all authors.
References
2. Capasso G, Di Gennaro CI, Della Ragione F, Manna C, Ciarcia R, Florio S, et al. In vivo effect of the natural antioxidant hydroxytyrosol on cyclosporine nephrotoxicity in rats. Nephrol Dial Transplant. 2008; 23:1186–95.

3. Lim SW, Hyoung BJ, Piao SG, Doh KC, Chung BH, Yang CW. Chronic cyclosporine nephropathy is characterized by excessive autophagosome formation and decreased autophagic clearance. Transplantation. 2012; 94:218–25.

4. Shelton LM, Park BK, Copple IM. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013; 84:1090–5.

5. Jeong HS, Ryoo IG, Kwak MK. Regulation of the expression of renal drug transporters in KEAP1-knockdown human tubular cells. Toxicol In Vitro. 2015; 29:884–92.

6. Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009; 76:277–85.

7. Hur W, Gray NS. Small molecule modulators of antioxidant response pathway. Curr Opin Chem Biol. 2011; 15:162–73.

8. Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010; 31:90–9.

9. Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012; 32:687–726.

10. Copple IM. The Keap1-Nrf2 cell defense pathway: a promising therapeutic target? Adv Pharmacol. 2012; 63:43–79.
11. Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol. 2009; 136:4–16.

12. Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003; 361:477–85.

13. Cawood AL, Ding R, Napper FL, Young RH, Williams JA, Ward MJ, et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010; 212:252–9.

14. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007; 369:1090–8.

15. Wen YT, Dai JH, Gao Q. Effects of omega-3 fatty acid on major cardiovascular events and mortality in patients with coronary heart disease: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014; 24:470–5.

16. van der Heide JJ, Bilo HJ, Donker JM, Wilmink JM, Tegzess AM. Effect of dietary fish oil on renal function and rejection in cyclosporine-treated recipients of renal transplants. N Engl J Med. 1993; 329:769–73.

17. Sakai C, Ishida M, Ohba H, Yamashita H, Uchida H, Yoshizumi M, et al. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced NA damage in vascular endothelial cells. PLoS One. 2017; 12:e0187934.
18. Shin DH, Park HM, Jung KA, Choi HG, Kim JA, Kim DD, et al. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic Biol Med. 2010; 48:1051–63.

19. Ishikado A, Morino K, Nishio Y, Nakagawa F, Mukose A, Sono Y, et al. 4-Hydroxy hexenal derived from docosahexaenoic acid protects endothelial cells via Nrf2 activation. PLoS One. 2013; 8:e69415.

20. Kusunoki C, Yang L, Yoshizaki T, Nakagawa F, Ishikado A, Kondo M, et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2013; 430:225–30.

21. Yang CW, Ahn HJ, Kim WY, Li C, Jung JY, Yoon SA, et al. Synergistic effects of mycophenolate mofetil and losartan in a model of chronic cyclosporine nephropathy. Transplantation. 2003; 75:309–15.

22. An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol. 2009; 297:F895–903.

23. Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011; 365:327–36.

24. de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013; 369:2492–503.

25. Kong W, Fu J, Liu N, Jiao C, Guo G, Luan J, et al. Nrf2 deficiency promotes the progression from acute tubular damage to chronic renal fibrosis following unilateral ureteral obstruction. Nephrol Dial Transplant. 2018; 33:771–83.

26. Hasnan J, Yusof MI, Damitri TD, Faridah AR, Adenan AS, Norbaini TH. Relationship between apoptotic markers (Bax and Bcl-2) and biochemical markers in type 2 diabetes mellitus. Singapore Med J. 2010; 51:50–5.
27. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–19.
28. Zhang L, Xu C, Hu W, Wu P, Qin C, Zhang J. Anti-inflammatory effects of Lefty-1 in renal tubulointerstitial inflammation via regulation of the NF-κB pathway. Int J Mol Med. 2018; 41:1293–304.

Fig. 1.
Morphological changes in the kidneys in the three groups. Periodic acid-Schiff-stained sections were examined using a light microscope (original magnification, ×200). Treatment of rats with cyclosporine for 4 weeks induced interstitial fibrosis, inflammatory cell infiltration, and tubular atrophy (B) compared to the controls (A). Omega-3 fatty acid supplementation decreased the pathologic changes (C).
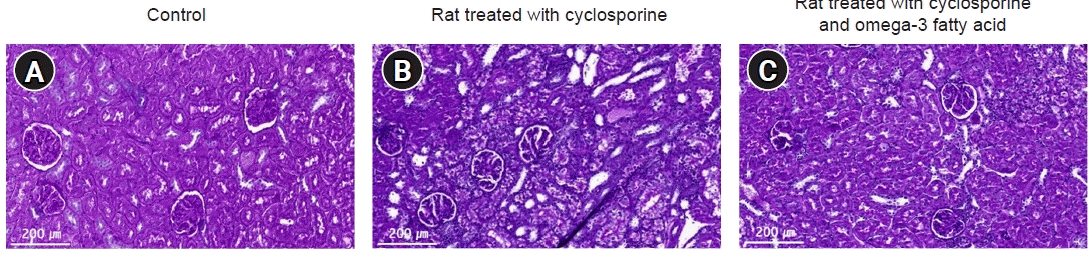
Fig. 2.
Expression of caspase-3 (A) and caspase-7 (B) in the kidneys of cyclosporine A (CsA)-induced rats detected by Western blots. Compared to the control group, increased expression of caspase-3 and caspase-7 was shown in the kidneys of the CsA group, and omega-3 fatty acid (O-3 FA) supplementation reversed these effects. *p<0.05, compared to control, **p<0.05, compared to the CsA group.
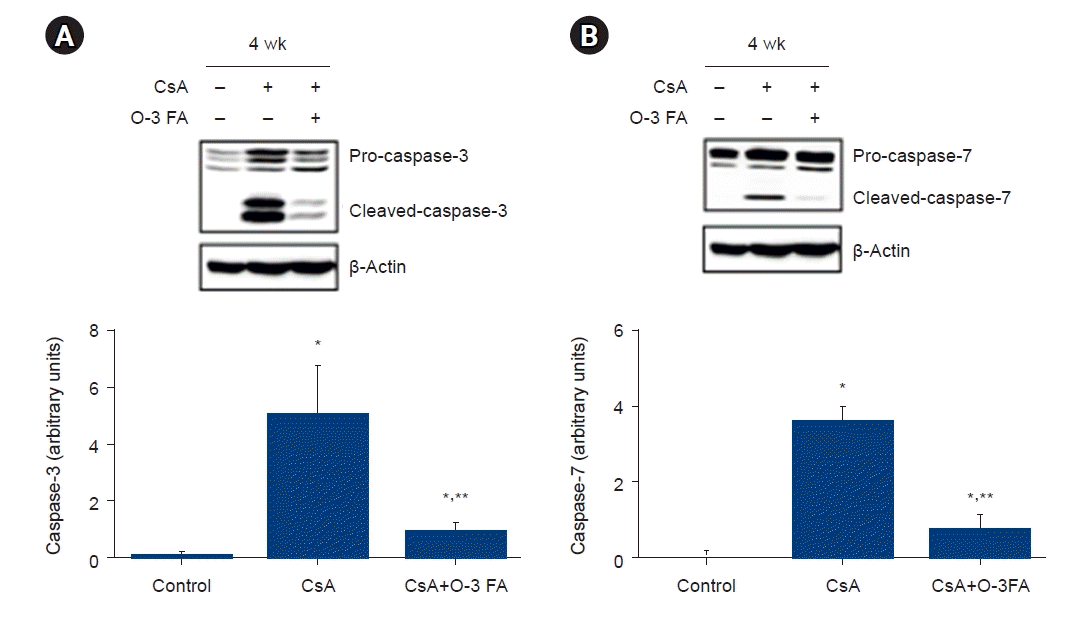
Fig. 3.
Bax and Bcl-2 expression in the kidneys of cyclosporine A (CsA)-induced rats. Compared to the control group, CsA treatment increased the expression of pro-apoptotic Bax (A) and attenuated the expression of anti-apoptotic Bcl-2 (B). Omega-3 fatty acid (O-3 FA) supplementation attenuated the upregulation of Bax and increased that of the anti-apoptotic protein Bcl-2. *p<0.05 compared to control, **p<0.05 compared to the CsA group.
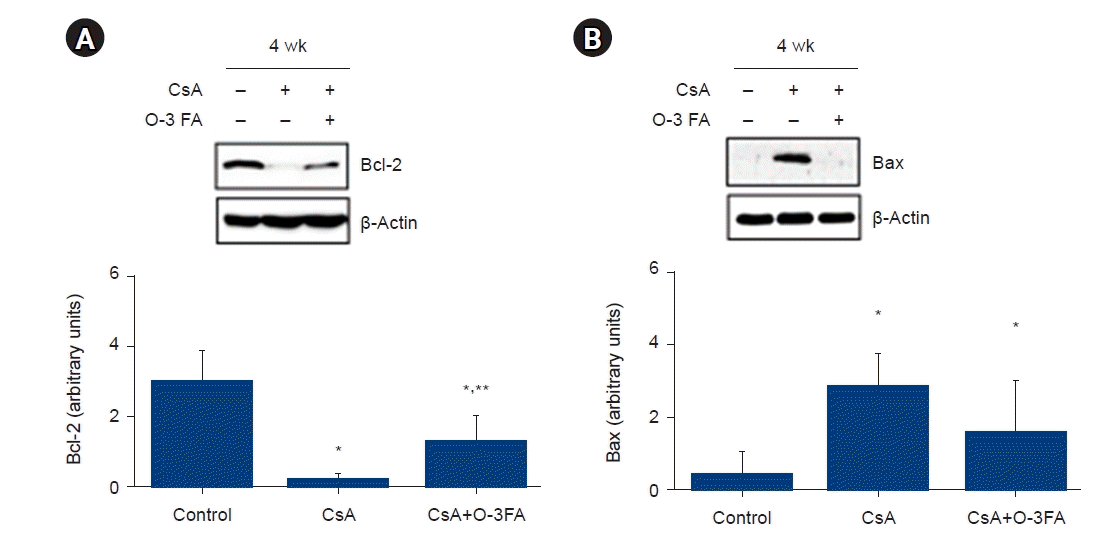
Fig. 4.
ED-1 and IĸB expression in the kidneys of cyclosporine A (CsA)-induced rats. Compared to the control group, significantly increased expression of ED-1 (A) and IĸB (B) was observed in CsA-treated rats, which was ameliorated by omega-3 fatty acid (O-3 FA) treatment. *p<0.05 compared to control, **p<0.05 compared to the CsA group.
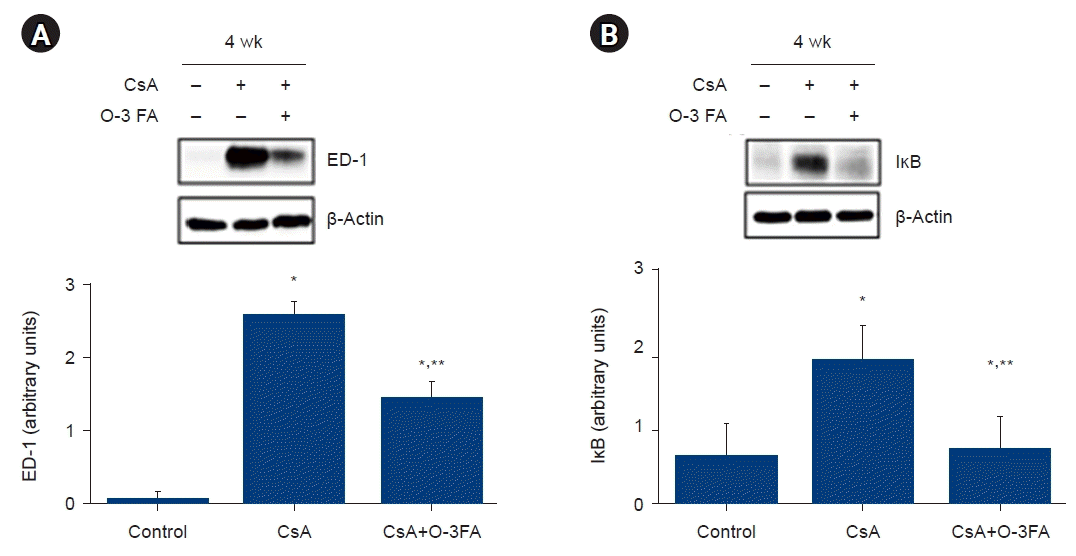
Fig. 5.
Smads2/3, Smad4, and transforming growth factor-β1 (TGF-β) expression in the kidneys of cyclosporine A (CsA)-induced rats. Compared to the control group, CsA treatment significantly increased the expression of TGF-β1, Smad2/3, and Smad4. Omega-3 fatty acid (O-3 FA) supplementation significantly prevented the upregulation of TGF-β1, Smad2/3, and Smad4. *p<0.05 compared to control, **p<0.05 compared to the CsA group.
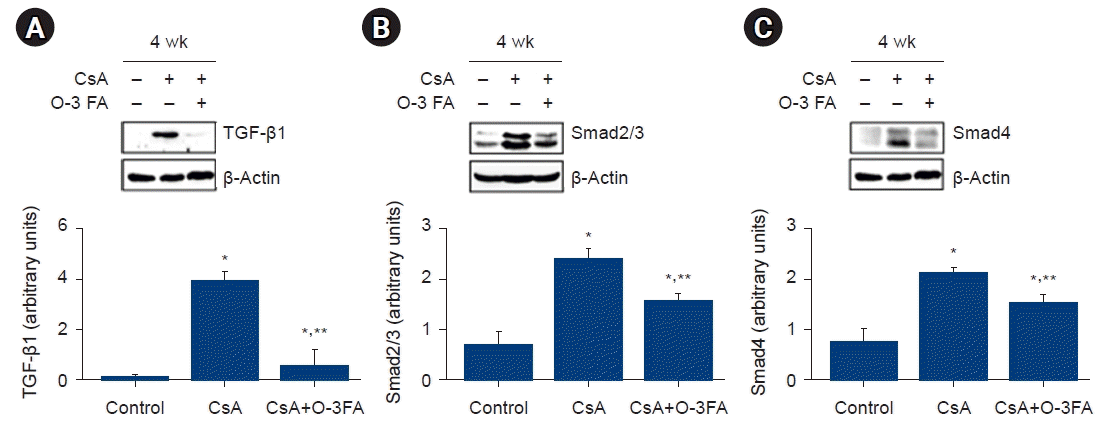
Fig. 6.
Nuclear factor erythroid 2-related factor 2 (Nrf2) expression in the kidney of cyclosporine A (CsA)-induced rats detected by Western blots. (A) Compared to the control group, Nrf2 expression was downregulated in the kidneys of the CsA group and upregulated by omega-3 fatty acid (O-3 FA) supplementation. (B) Immunohistochemically stained specimens from the control group. Nrf2 was mainly expressed in the tubules of the normal control rats. (C) Nrf2 expression was increased in the kidneys of CsA-induced rats. (D) Nrf2 expression was decreased by O-3 FA supplementation in the kidney of CsA-induced rats. *p<0.05 compared to control, **p<0.05, compared to the CsA group.
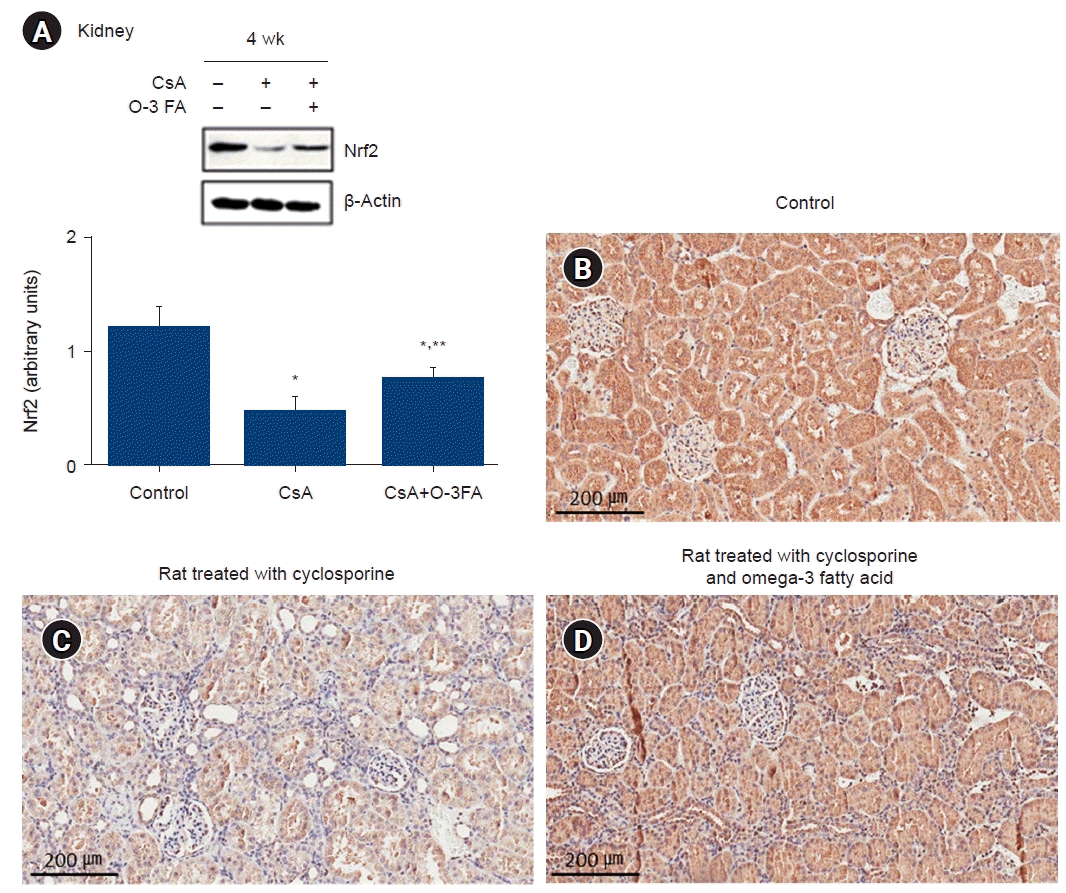
Table 1.
Biochemical characteristics in each group
| Characteristic | Control (n=6) | Cyclosporine (n=6) | Cyclosporine with omega-3 fatty acids (n=6) | p-value |
|---|---|---|---|---|
| Final body weight (g) | 433.67±8.62 | 335.81±1.82* | 344.06±9.80* | <0.001 |
| Blood urea nitrogen (mg/dL) | 16.15±2.78 | 56.08±15.60* | 50.53±7.50* | <0.001 |
| Creatinine (mg/dL) | 0.40±0.03 | 0.55±0.12* | 0.54±0.29* | - |
| Cyclosporine (ng/mL) | - | 2,732±198 | 2,587±320 | 0.469 |
| Total cholesterol (mg/dL) | 67.44±16.33 | 108.50±3.83* | 92.50±9.88* | <0.001 |
| Triglyceride (mg/dL) | 111.9±40.8 | 349.8±122.4* | 305.3±43.6* | <0.001 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download