Abstract
The coronavirus disease 2019 (COVID-19) has been a major public health emergency worldwide. Vaccines were rapidly developed and approved to prevent the spread of viral infection. However, various side effects of the COVID-19 messenger RNA (mRNA) vaccines have been reported after their commercialization. A 24-year-old man visited our emergency department with polyuria and polydipsia that occurred after he received a COVID-19 mRNA vaccine 10 days beforehand. The initial laboratory findings showed very low urine osmolality with hyperosmolar hypernatremia. Based on these findings, diabetes insipidus was suspected, and sella magnetic resonance imaging showed an enlarged pituitary gland and the absence of posterior pituitary higher intensity. After 12 hours of using oral desmopressin acetate, urine volume decreased, and after 5 days of administration, serum electrolyte and serum osmolality improved. This case report of diabetes insipidus occurring after vaccination with the BNT162b2 mRNA COVID-19 vaccine is presented as a reminder that close monitoring is necessary for patients with polyuria and polydipsia after vaccination.
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a major worldwide public health emergency. To prevent infection, many vaccines rapidly tested in small groups and emergency use authorization was given [1].
Phase 3 clinical trials of the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer-BioNTech) indicated efficacy against COVID-19 infection, and showed that the vaccine had an acceptable safety profile [2]. However, this clinical trial included only 43,548 participants; thus, it was difficult to identify less common adverse events. After post-marketing of the COVID-19 mRNA vaccine, side effects were reported including myocarditis [3,4], cerebral venous sinus thrombosis [5], immune thrombocytopenic purpura [6] and new-onset autoimmune diseases [7].
Here, we report the case of diabetes insipidus after administration of the BNT162b2 mRNA vaccine in a 24-year-old male patient.
Ethical statements: This report was exempted from review by the Institutional Review Board (IRB) of Gyeongsang National University Hospital (IRB No. 2023-01-001). Written informed consent was obtained from the patients to participate in the study.
A 24-year-old man visited the emergency department of our hospital with polyuria and an electrolyte abnormality. He had no medical history, and was otherwise in good health (September 2021). Symptoms of polyuria and polydipsia developed 10 days after he received the COVID-19 mRNA vaccine. At that time, blood analysis was performed at a local hospital and no abnormalities, including in glucose and electrolyte levels, were detected. However, the symptoms were persistent. He visited another hospital and was referred to our institution for further evaluation of the electrolyte imbalance. He had an increased urine output of more than 6–8 L/day at that time (November 2021). A COVID-19 test was negative.
Physical examination on admission revealed a blood pressure of 140/80 mmHg, heart rate of 110/min, body temperature of 37.8 ℃ and alert mental status.
The laboratory findings at admission were as follows: hemoglobin, 17.8 g/dL; white blood cell count, 8.56×109/L (segmented neutrophils, 47%; lymphocytes, 40%); platelets, 239×109/L; protein, 7.1 g/dL; albumin, 4.7 g/dL; alkaline phosphatase, 72 U/L; aspartate aminotransferase, 88 U/L; alanine aminotransferase, 98 U/L; blood urea nitrogen, 12.8 mg/dL; and creatinine, 1.06 mg/dL. His serum electrolytes and blood gas parameters were as follows: sodium, 175.6 mM/L; potassium, 3.9 mM/L; chloride, 140 mM/L; osmolality, 357 mOs/kg; calcium, 9.4 mg/dL; phosphorus, 2.5 mg/dL; pH, 7.38; pCO2, 46 mmHg; pO2, 81 mmHg; and bicarbonate, 27 mM/L. Finally, his urinalysis results were as follows: osmolality, 112 mOs/kg (normal range, 300–900 mOs/kg); and density, 1.002.
Diabetes insipidus was suspected based on the test results. Further hormonal testing was performed, and the results were as follows: thyroid-stimulating hormone, 1.58 (0.27–4.2) μIU/mL; T3, 152 (80–200) ng/dL; free T4, 1.4 (0.93–1.7) ng/dL; adrenocorticotropic hormone, 72 pg/mL; cortisol, 22.2 µg/dL; follicle-stimulating hormone, 0.87 UI/L; luteinizing hormone, 2.57 UI/L; insulin-like growth factor-1, 243 (88–209) ng/mL; prolactin, 34.7 (4.0–15.2) µg/L; and arginine vasopressin, 3.0 pg/mL.
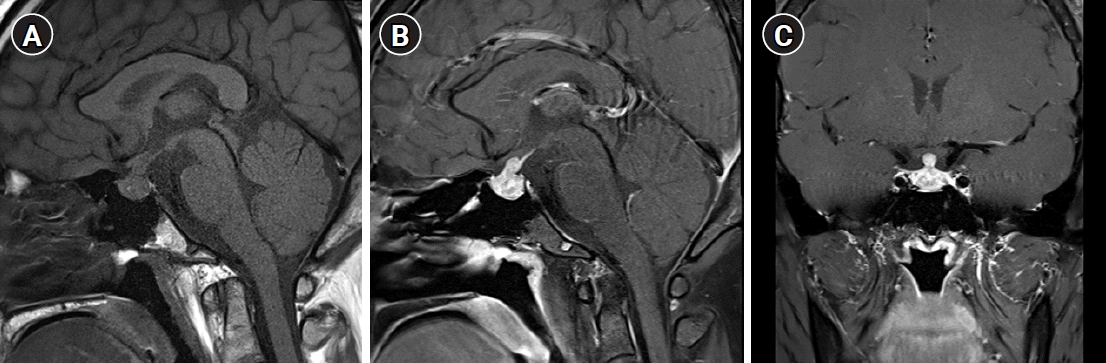
Magnetic resonance imaging (MRI) of the pituitary gland revealed an enlarged pituitary gland and stalk, with no posterior pituitary hyperintensity (Fig. 1).
Diabetes insipidus was diagnosed based on these findings. The patient was started on oral 0.2 mg desmopressin acetate (three times per day). Although no pituitary biopsy was conducted, other plausible causes of central diabetes insipidus (CDI) were ruled out, including IgG4-related disease and autoimmune diseases.
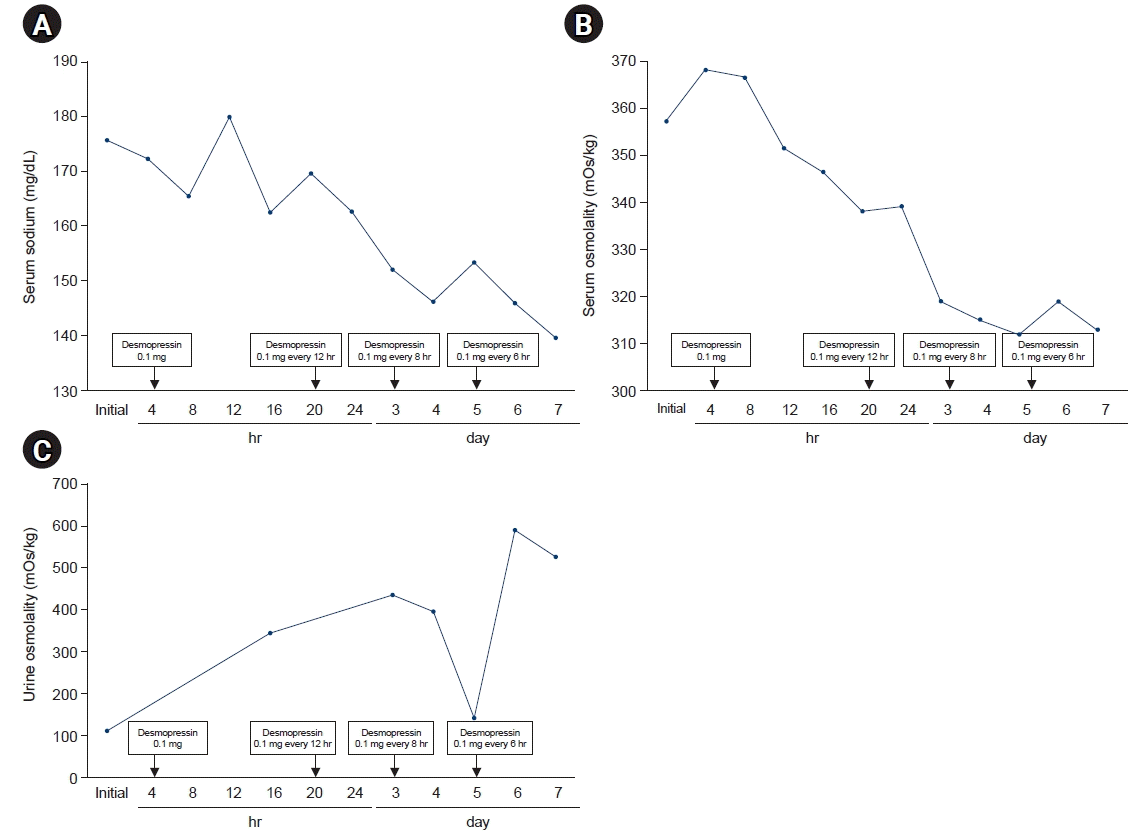
After 12 hours of using oral desmopressin acetate, the urine volume decreased and the urine osmolality increased rapidly. The serum electrolytes and serum osmolality were improved after 5 days of administration (sodium, 139.3 mM/L; potassium, 4.4 mM/L; chloride, 98 mM/L; osmolality, 290 mOs/kg; 24-hour urine volume, 2,300 mL; urine osmolality, 437 [300–900] mOs/kg; urine density, 1.011) (Fig. 2).
At the last visit in May 2022, 7 months after the first visit, the patient was taking 0.2 mg desmopressin acetate three times per day and had no polydipsia or polyuria. MRI of the pituitary gland 3 months later showed that the pituitary stalk was still enlarged.
We report a case of diabetes insipidus developing after BNT162b2 COVID-19 mRNA vaccination, suggesting that it is necessary to monitor patients after mRNA vaccinations. CDI is characterized by hypotonic polyuria due to impaired AVP secretion from the posterior pituitary [8]. First, we confirmed the presence of hypotonic polyuria. The most commonly used test for diagnosing diabetes insipidus is the water deprivation test. However, we did not conduct this test because the sodium and osmolality levels of our patient were very high. Instead, we diagnosed diabetes insipidus after a therapeutic trial of desmopressin, which eliminated the polyuria and lowered the plasma osmolarity/sodium levels. We also measured the plasma AVP level, which was 3.0 pg/mL; the serum osmolality was 357 mOs/kg.
A several cases of side effects related to the pituitary gland after vaccination with SARS-CoV-2 were reported, and the types of vaccines received were different [9-11]. In this case, there was one case of CDI after BNT162b2 COVID-19 mRNA vaccine in a healthy person, and the case was reported where symptoms occurred 2 days after vaccination and persisted for more than 3 months [11]. Unlike previously reported case, this case is a young male who presented with a relatively rapid and severe electrolyte imbalance.
Hypophysitis is a heterogeneous condition that leads to inflammation of the sella and/or suprasellar region, potentially resulting in hormonal deficiencies and/or mass effects [12]. The etiology of primary hypophysitis is not clearly known, but majority of hypophysitis cases have an autoimmune etiology [13]. Secondary hypophysitis is caused by a variety of causes, including not only systemic inflammatory diseases but also tumors or cysts in pituitary lesions, infections, and various drugs. Various hypotheses have been proposed for the pathophysiological mechanism of post-vaccination hypophysitis, including autoimmune and inflammatory syndromes induced by vaccine adjuvants, hyper-stimulation of the immune system against vaccine components, and systemic inflammatory response [14]. However, since a pathological analysis of the pituitary gland must be performed for a comprehensive pathophysiological understanding, it is considered very difficult to directly prove the pituitary gland biopsy because of the risks.
Chen et al. [7] showed that new-onset autoimmune diseases are being increasingly reported after COVID-19 vaccination. In our case, there were no specific laboratory findings suggesting other causes of CDI, nor was there a history of drug use. Therefore, hypothalamic damage and inflammatory response occurred after vaccination, and CDI caused by primary hypophysitis was most suspected.
CDI is a rare side effect of mRNA vaccination; aside from our case, there have been only several previous reports. Physicians should be aware of the potential for adverse events in relation to mRNA vaccination.
References
1. U.S. Food and Drug Administration (FDA). Emergency use authorization: PfizerBioNTech COVID-19 vaccine [Internet]. U.S. FDA; c2021 [cited 2023 May 18]. https://www.fda.gov/vaccines-blood-biologics/coronavirus-covid-19-cber-regulated-biologics/pfizer-biontech-covid-19-vaccines.
2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383:2603–15.

3. Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021; 385:2140–9.

4. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021; 6:1202–6.

5. Krzywicka K, Heldner MR, Sanchez van Kammen M, van Haaps T, Hiltunen S, Silvis SM, et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021; 28:3656–62.

6. King ER, Towner E. A case of immune thrombocytopenia after BNT162b2 mRNA COVID-19 vaccination. Am J Case Rep. 2021; 22:e931478.

7. Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022; 165:386–401.

8. Garrahy A, Moran C, Thompson CJ. Diagnosis and management of central diabetes insipidus in adults. Clin Endocrinol (Oxf). 2019; 90:23–30.

9. Murvelashvili N, Tessnow A. A case of hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Investig Med High Impact Case Rep. 2021; 9:23247096211043386.

10. Bouca B, Roldao M, Bogalho P, Cerqueira L, Silva-Nunes J. Central diabetes insipidus following immunization with BNT162b2 mRNA COVID-19 vaccine: a case report. Front Endocrinol (Lausanne). 2022; 13:889074.
11. Ankireddypalli AR, Chow LS, Radulescu A, Kawakami Y, Araki T. A case of hypophysitis associated with SARS-CoV-2 vaccination. AACE Clin Case Rep. 2022; 8:204–9.

Fig. 1.
Magnetic resonance imaging of the pituitary gland. (A) Sagittal pre-contrast T1 image showing an enlarged pituitary gland and stalk, with no posterior pituitary hyperintensity. (B) Sagittal and (C) coronal post-contrast T1 images showing an enlarged pituitary gland and stalk, with heterogeneous enhancement.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download