Abstract
An aging population and changes in dietary habits have increased the incidence of diabetes, resulting in complications such as diabetic foot ulcers (DFUs). DFUs can lead to serious disabilities, substantial reductions in patient quality of life, and high financial costs for society. By understanding the etiology and pathophysiology of DFUs, their occurrence can be prevented and managed more effectively. The pathophysiology of DFUs involves metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and angiopathy. The processes by which hyperglycemia causes peripheral nerve damage are related to adenosine triphosphate deficiency, the polyol pathway, oxidative stress, protein kinase C activity, and proinflammatory processes. In the context of hyperglycemia, the suppression of endothelial nitric oxide production leads to microcirculation atherosclerosis, heightened inflammation, and abnormal intimal growth. Diabetic neuropathy involves sensory, motor, and autonomic neuropathies. The interaction between these neuropathies forms a callus that leads to subcutaneous hemorrhage and skin ulcers. Hyperglycemia causes peripheral vascular changes that result in endothelial cell dysfunction and decreased vasodilator secretion, leading to ischemia. The interplay among these four preceding pathophysiological factors fosters the development and progression of infections in individuals with diabetes. Charcot neuroarthropathy is a chronic and progressive degenerative arthropathy characterized by heightened blood flow, increased calcium dissolution, and repeated minor trauma to insensate joints. Directly and comprehensively addressing the pathogenesis of DFUs could pave the way for the development of innovative treatment approaches with the potential to avoid the most serious complications, including major amputations.
Diabetic foot ulcers (DFUs) are ulcers that arise on the feet of individuals with diabetes and are a major concern. These ulcers stem from the deterioration of the skin or mucosal tissue on the feet and are particularly susceptible to exacerbation by conditions such as diabetic neuropathy and peripheral vascular disease. Upon occurrence, DFUs can culminate in foot amputation, inflicting a substantial psychological burden on patients. This unfortunate outcome can lead to a reduction in one's daily activities, culminating in a decline in both physical capabilities and social engagement, leading to a serious reduction in the patient's quality of life and high financial costs for society [1].
As the average age of the population increases worldwide, the number of patients with diabetes, and accordingly, the number of patients with DFUs, is also increasing. The lifetime prevalence of DFUs in the population with diabetes is 15% to 25%, and the recurrence rate of DFUs within 5 years is high, ranging from 50% to 70% [2-4].
Understanding the pathophysiology of DFUs is crucial for effective management and prevention, facilitating early diagnosis, informed decision-making, personalized treatment, improved wound healing, fewer complications, preventive actions, and research advancements.
The pathophysiology of diabetic ulcers involves metabolic causes, neuropathy, angiopathy, and changes in the immune system. The interaction between metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and diabetic angiopathy promotes the development and progression of diabetic foot infections (DFIs) and may lead to diabetic neuroarthropathy (Figs. 1, 2).
Diabetes affects epineural microvessels and reduces blood supply to the nerves of patients with diabetes [5]. Apart from the vascular supply, the peculiar anatomy of the peripheral nervous system may explain the predisposition of its most distal parts to diabetes. The axon is covered with Schwann cells, but the distal axon is too weak because the neuronal cell body is relatively small compared to the very long axon neurite. Therefore, distal axons are vulnerable when diabetes affects nerves [6].
The processes by which hyperglycemia causes peripheral nerve damage are related to adenosine triphosphate (ATP) deficiency, the polyol pathway, oxidative stress, protein kinase C (PKC) activity, and proinflammatory processes.
An insufficient ATP supply hampers axonal transport, particularly in mitochondria-rich axons that provide nerve energy, thus promoting axonal injury and diabetic neuropathy. The inability to counter excessive oxidative stress due to inadequate ATP levels damages the axons during hyperglycemia, causing axonal degeneration or apoptosis [7]. Oxidative stress negatively affects multiple biochemical pathways.
The polyol pathway involves the conversion of glucose to sorbitol by aldose reductase (AR) and the subsequent conversion of sorbitol to fructose by sorbitol dehydrogenase. In diabetes, elevated glucose levels boost the affinity of AR for glucose, leading to increased sorbitol production. Accumulated sorbitol reduces Na+K+-ATPase activity, thereby diminishing nerve cell reserves and conduction velocities. The hyperglycemia-induced polyol pathway also increases oxidative stress due to nicotinamide adenine dinucleotide phosphate (NADPH) depletion via the pentose phosphate pathway, which is essential for glutathione generation [8]. Excess fructose accelerates glycation and NADPH consumption and exacerbates intracellular oxidative stress [9]. This disturbance results in decreased antioxidant levels and increased production of reactive oxygen species (ROS), which play pivotal roles in diabetes-related complications. The activation of AR increases polyol flux and causes neuropathy; however, neuropathic changes have also been observed in AR-deficient diabetic mice [10]. Further research is required to establish a direct link between AR and diabetic neuropathy.
PKC is a member of the serine/threonine protein kinase family [11] and is involved in various cellular responses associated with diabetes [12]. Hyperglycemia triggers glycolysis and activates PKC [13]. Vascular dysfunction caused by PKC activation promotes diabetic microvascular complications, which primarily alter blood flow [14], extracellular matrix synthesis, basement membrane thickening [15], vascular permeability [16], and angiogenesis [17].
Low-grade intraneural inflammation is an aspect of diabetic neuropathy. Systemic proinflammatory activity in human sensorimotor diabetic neuropathy has recently been reported [18]. This mild inflammatory process is a common terminal pathway in diabetic neuropathy and is associated with the degeneration of intraepidermal nerve fibers.
In the context of hyperglycemia, the suppression of endothelial nitric oxide (NO) production inhibits NO synthase, leading to an elevated level of ROS, notably superoxide radicals. This heightened ROS level subsequently triggers an increase in hydrogen peroxide concentrations. Consequently, highly reactive hydroxyl radicals can be formed that cause cellular damage. The combined action of NO and superoxide results in the production of peroxynitrite, which, in turn, affects endothelial vasodilation and mediates lipid peroxidation. This process sets the stage for heightened concentrations of low-density lipoproteins, followed by the development of microcirculation atherosclerosis, elevated inflammation, abnormal intimal growth, platelet aggregation, and thrombosis [19].
Hyperglycemia contributes to excess hydrogen peroxide, intensifying oxidative stress and related products [20]. These products stimulate the generation of advanced glycation end-products (AGEs) [21]. Decoupling of NO synthase reduces NO production, resulting in impaired wound healing [22]. In the context of wound healing, the controlled generation and elimination of ROS are vital. However, diabetic wounds exhibit elevated ROS levels, which further impede the healing process. The heightened presence of ROS not only slows wound healing but also leads to excessive oxidative stress.
Peripheral neuropathy is the most common intractable complication of diabetes [23,24]. More than 60% of DFUs result from an underlying neuropathy [25]. The duration of diabetes and glycated hemoglobin levels are strongly associated with the prevalence of neuropathy [26,27].
Blood supply to the peripheral nerves is insufficient, blood flow is easily damaged, and automatic regulation of blood flow is impaired. These anatomical features enable us to understand why peripheral nerve neuropathy differs from other complications [28]. These features make peripheral nerves vulnerable to ischemia.
In diabetic neuropathy, damaged nerve endings lead to pain perception due to disrupted action potentials, inducing hyperexcitability [29]. Upregulated voltage-gated sodium channels (Nav), including Nav1.3 and Nav1.7, in diabetic animals influence diabetic neuropathy progression, while reduced Nav1.8 and Nav1.6, along with abnormal phosphorylation of Nav1.6, Nav1.7, and Nav1.8, contribute to nociceptive nerve fiber irregularities [30,31]. Patients with diabetic neuropathy display increased nodal Na+ currents that intensify peripheral nerve hyperexcitability.
Altered K+ voltage-dependent channels (Kv) affect resting membrane potential and increase neuronal excitability [32]. Reduced Kv currents in diabetic rats elevate excitability and peptide release, and these factors could contribute to peripheral nerve hyperexcitability in diabetes.
The development of neuropathy in affected patients results from hyperglycemia-induced metabolic abnormalities [33,34]. Diabetic neuropathy affects the sensory, motor, and autonomic nervous systems.
The evidence of sensory neuropathy includes hyperalgesia, paresthesia, and allodynia. Sensory neuropathy causes loss of protective sensations. As a result, the risk for trauma is significantly elevated [35,36] and injuries often go unnoticed for weeks. Motor neuropathy can manifest as atrophy of the small foot muscles, resulting in malpositioning of the toes (claw toe) [37]. These muscle changes can cause foot deformities leading to biomechanical abnormalities. Glycosylation of tendons induces stiffness and shortening, potentially giving rise to foot deformities, such as claw toes and hammer toes, along with Achilles tendon stiffening, which elevates the pressure on the forefoot [38]. The combination of sensory and motor peripheral neuropathy results in an unequal foot load with an unsteady gait. Over time, hyperkeratosis develops because of neuropathy and an elevated plantar pressure load. Autonomic neuropathy reduces the function of sweat and sebaceous glands of the feet, resulting in dry skin and fissures. Furthermore, it diminishes the neuroinflammatory responses to noxious stimuli [39]. Consequently, the natural ability of the foot to moisturize is lost, and the skin becomes more susceptible to injury and infection. The interaction between sensory, motor, and autonomic neuropathy can form a callus in the foot. After repeated exposure to external or minor trauma, skin ulcers form through subcutaneous hemorrhage (Fig. 3) [40].
Hyperglycemia causes endothelial damage, dyslipidemia, and increased platelet viscosity and activity, leading to atherosclerosis. Hyperglycemia also causes peripheral vascular changes, resulting in endothelial cell dysfunction and decreased vasodilator secretion [25,41]. Peripheral vascular constriction and hypercoagulation lead to ischemia, which increases the risk of skin ulcers. Peripheral arterial disease is an important cause of foot ulcers in approximately 50% of cases [42]. An insufficient peripheral blood supply delays wound healing and exacerbates infections [43].
In diabetic neuropathy, the perception of pain arises from impaired nerve endings that disrupt the normal progression of action potentials. Atherosclerosis is the primary contributor to vascular mortality in type 2 diabetes. This process is driven by endothelial cell dysfunction, a consequence of factors like hypertension, insulin resistance, and hyperglycemia. The resulting dysfunction not only hampers the production of NO by endothelial cells but also triggers signals that encourage the proliferation of vascular smooth muscle cells through the mitogen-activated protein kinase pathway. Elevated glucose levels counteract the vasodilatory effects of NO and increase the levels of antifibrinolytic and prothrombotic molecules [4].
The intricate relationship among insulin resistance, hyperglycemia, and dyslipidemia underscores the nature of diabetes. Through extensive research, four interconnected pathways have been revealed, shedding light on the connection between hyperglycemia and endothelial cell damage, resulting in shared biochemical abnormalities. Elevated glucose exposure spurs the production of mitochondrial superoxide, thereby influencing downstream pathways including the hexosamine and PKC pathways. Hyperglycemia-induced mitochondrial generation of ROS affects both endothelial cells and platelets, inciting platelet aggregation and cytokine release [4,44].
In the context of diabetes, targeting the excessive production of mitochondrial ROS is promising for addressing vascular dysfunction. Gliclazide, a sulfonylurea, possesses antioxidant and anti-aggregation properties that can potentially enhance vascular regulation and mitigate oxidative stress. It effectively interferes with AGE-induced vascular endothelial growth factor expression while modulating signaling pathways intricately linked to diabetic angiopathy [45].
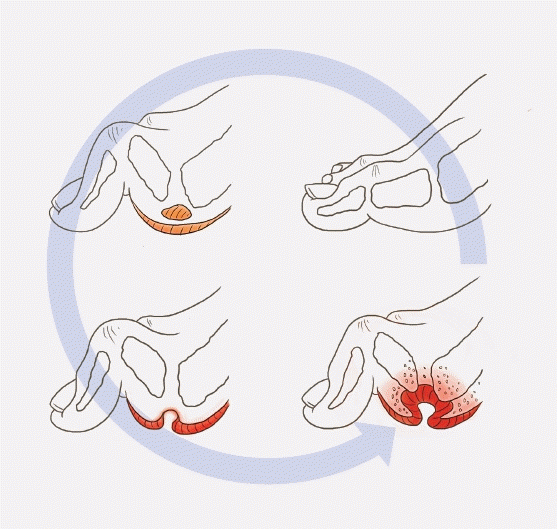
The interplay of metabolic factors, immunopathy, diabetic neuropathy, and diabetic angiopathy promotes the development and progression of infections, ischemic ulcers, and gangrene in diabetic individuals, potentially culminating in amputation (Fig. 4) [46,47].
A range of microorganisms can trigger DFIs (Fig. 3) [40], with Staphylococcus aureus being the most prevalent. Among DFIs, methicillin-resistant S. aureus (MRSA) is found in 16.78% to 30% of cases [48]. Although MRSA infections do not appear to affect mortality, they are associated with increased hospitalization rates and increased risks of limb amputation [49]. Amputation, as a preventive measure against the spread of infection, was shown to extend life expectancy by 2 years in half of the studied subjects with diabetes [50,51]. Nevertheless, the survival rate of patients with diabetes and ulcerative infections remains at only 56% 5 years after the initial onset of ulcers [51]. Collectively, these findings underscore the urgency of enhanced ulcer prevention and prompt diagnosis of DFIs.
Charcot neuroarthropathy, commonly referred to as Charcot foot, is a chronic and progressive degenerative arthropathy that arises from disrupted sensory innervation of the affected joint. This insidious and destructive condition primarily affects the foot bones, leading to deformities that can trigger ulcer formation and subsequent disabilities. Charcot foot development is characterized by joint subluxation and dislocation, bone osteolysis, fragmentation, and soft-tissue edema [47].
Deterioration of the autonomic nervous system in individuals with diabetes mellitus leads to increased local blood supply, resulting in elevated resting blood flow compared to that in individuals who are non-diabetic. This heightened blood flow combined with increased calcium dissolution promotes osteoclastic bone activity and damage. Another theory suggests that repeated minor trauma to the insensate joints contributes to fracture and disintegration.
In patients with Charcot foot, proinflammatory proteins exhibit distinct expression patterns under modulated stimulation. The production of proinflammatory cytokines, such as tumor necrosis factor-α and interleukin (IL)-1β, triggers uncontrolled osteolysis. These cytokines upregulate the expression of receptor activator of nuclear factor (NF)-κB ligand (RANKL), which in turn promotes osteoclast maturation through NF-κB activation, while the anti-inflammatory peptides IL-4 and IL-10 are downregulated.
A hallmark deformity associated with Charcot foot is midfoot collapse, often termed the “rocker-bottom” foot deformity. Hallux valgus and loose bodies in the joint cavity may also be present. These deformities increase susceptibility to recurrent ulcerations [52].
Hyperglycemia induces diabetic ulcers through several processes. The interactions among metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and diabetic angiopathy promote the development and progression of DFIs and may lead to diabetic neuroarthropathy. A direct and detailed approach to understanding the pathogenesis of DFUs will enable the development of new treatment methods and prevent the worst outcomes of diabetic ulcers such as major amputation.
References
1. Sumpio BE. Contemporary evaluation and management of the diabetic foot. Scientifica (Cairo). 2012; 2012:435487.

2. Boulton AJ. Hyperbaric oxygen in the management of chronic diabetic foot ulcers. Curr Diab Rep. 2010; 10:255–6.

3. Boulton AJ, Armstrong DG. Diabetic foot and ulceration: epidemiology and pathophysiology. In : Falabella A, Kirsner R, editors. Wound healing. Boca Raton: CRC Press:;2005.
4. Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000; 97:12222–6.

5. Grover-Johnson NM, Baumann FG, Imparato AM, Kim GE, Thomas PK. Abnormal innervation of lower limb epineurial arterioles in human diabetes. Diabetologia. 1981; 20:31–8.

6. Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig. 2011; 2:18–32.

7. Fernyhough P, McGavock J. Mechanisms of disease: mitochondrial dysfunction in sensory neuropathy and other complications in diabetes. Handb Clin Neurol. 2014; 126:353–77.
8. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014; 18:1–14.

9. Ollendorf DA, Kotsanos JG, Wishner WJ, Friedman M, Cooper T, Bittoni M, et al. Potential economic benefits of lower-extremity amputation prevention strategies in diabetes. Diabetes Care. 1998; 21:1240–5.

10. Yagihashi S, Yamagishi SI, Mizukami H, Ogasawara S, Chung S, Chung SK. Escape phenomenon from polyol pathway to other metabolic cascades may underlie nerve conduction delay in severely hyperglycemic AR-deficient mice (abstract No. 775-P). Abstract presented at: American Diabetes Association, 68th Scientific Sessions. 2008 June 6-10; San Francisco. American Diabetes Association;2008.
11. Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012; 11:937–57.

12. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992; 258:607–14.

14. Schambelan M, Blake S, Sraer J, Bens M, Nivez MP, Wahbe F. Increased prostaglandin production by glomeruli isolated from rats with streptozotocin-induced diabetes mellitus. J Clin Invest. 1985; 75:404–12.
15. Gutiérrez A, Contreras C, Sánchez A, Prieto D. Role of phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and protein kinase C (PKC) in calcium signaling pathways linked to the α1-adrenoceptor in resistance arteries. Front Physiol. 2019; 10:55.

16. Retamal JS, Grace MS, Dill LK, Ramirez-Garcia P, Peng S, Gondin AB, et al. Serotonin-induced vascular permeability is mediated by transient receptor potential vanilloid 4 in the airways and upper gastrointestinal tract of mice. Lab Invest. 2021; 101:851–64.

17. Moriya J, Ferrara N. Inhibition of protein kinase C enhances angiogenesis induced by platelet-derived growth factor C in hyperglycemic endothelial cells. Cardiovasc Diabetol. 2015; 14:19.
18. Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN): new aspects. Int J Mol Sci. 2021; 22:10835.

19. Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. 2007; 45:55–9.

20. Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010; 59:249–55.

21. Wu H, Cai L, de Haan JB, Giacconi R. Targeting oxidative stress in diabetic complications: new insights. J Diabetes Res. 2018; 2018:1909675.

23. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005; 28:956–62.
24. Strotmeyer ES, de Rekeneire N, Schwartz AV, Resnick HE, Goodpaster BH, Faulkner KA, et al. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: the health, aging and body composition study. J Am Geriatr Soc. 2009; 57:2004–10.

25. Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes. 2009; 27:52–8.

26. Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, et al. Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes? Diabetologia. 2009; 52:1279–89.

27. Pop-Busui R, Lu J, Lopes N, Jones TL; BARI 2D Investigators. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst. 2009; 14:1–13.

28. Kihara M, Weerasuriya A, Low PA. Endoneurial blood flow in rat sciatic nerve during development. J Physiol. 1991; 439:351–60.

29. Dickenson AH, Matthews EA, Suzuki R. Neurobiology of neuropathic pain: mode of action of anticonvulsants. Eur J Pain. 2002; 6(Suppl A):51–60.

30. Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, et al. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999; 82:2776–85.

31. He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010; 151:266–79.
32. Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005; 569:41–57.
33. Feldman EL, Russell JW, Sullivan KA, Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol. 1999; 12:553–63.

35. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004; 351:48–55.
37. Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. 2016; 17:917.
38. Trieb K. The Charcot foot: pathophysiology, diagnosis and classification. Bone Joint J. 2016; 98-B:1155–9.
39. Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006; 82:95–100.
40. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017; 376:2367–75.

41. Paraskevas KI, Baker DM, Pompella A, Mikhailidis DP. Does diabetes mellitus play a role in restenosis and patency rates following lower extremity peripheral arterial revascularization? A critical overview. Ann Vasc Surg. 2008; 22:481–91.

42. Janka HU, Standl E, Mehnert H. Peripheral vascular disease in diabetes mellitus and its relation to cardiovascular risk factors: screening with the doppler ultrasonic technique. Diabetes Care. 1980; 3:207–13.

43. Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004; 39(Suppl 2):S83–6.

44. Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001; 50:1491–4.

45. Mamputu JC, Renier G. Advanced glycation end products increase, through a protein kinase C-dependent pathway, vascular endothelial growth factor expression in retinal endothelial cells. Inhibitory effect of gliclazide. J Diabetes Complications. 2002; 16:284–93.
46. Bandyk DF. The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018; 31:43–8.

47. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K; International Working Group on the Diabetic Foot. Prevention and management of foot problems in diabetes: a Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab Res Rev. 2016; 32(Suppl 1):7–15.

48. Stacey HJ, Clements CS, Welburn SC, Jones JD. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: a meta-analysis. Acta Diabetol. 2019; 56:907–21.

49. Saltoglu N, Ergonul O, Tulek N, Yemisen M, Kadanali A, Karagoz G, et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int J Infect Dis. 2018; 70:10–4.

50. Icks A, Scheer M, Morbach S, Genz J, Haastert B, Giani G, et al. Time-dependent impact of diabetes on mortality in patients after major lower extremity amputation: survival in a population-based 5-year cohort in Germany. Diabetes Care. 2011; 34:1350–4.
51. Kerr M; Insight Health Economics. Foot care for people with diabetes: the economic case for change. National Health Service;UK: 2012.
52. Singh S, Pai DR, Yuhhui C. Diabetic foot ulcer-diagnosis and management. Clin Res Foot Ankle. 2013; 1:120.
Fig. 1.
Diagram of pathophysiological factors in diabetic foot. The interaction of metabolic dysfunction, diabetic immunopathy, diabetic neuropathy, and diabetic angiopathy promotes the development and progression of diabetic foot infections and may lead to diabetic neuroarthropathy.
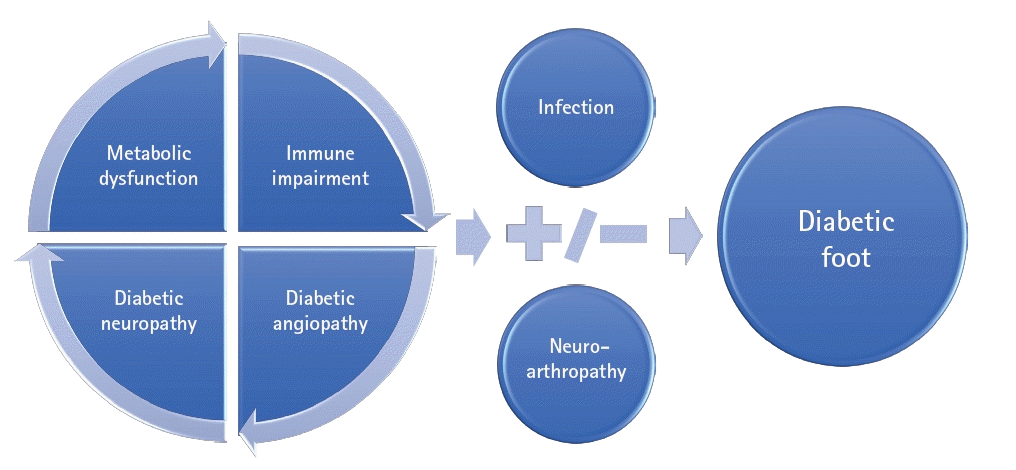
Fig. 2.
Pathological pathways in the progression of diabetic foot. A schematic diagram representing the pathophysiology of DFUs via different pathological pathways. Endothelial dysfunction, ischemia, neuroarthropathy, and impaired immunology contribute to the pathophysiology of DFUs. ATP, adenosine triphosphate; PKC, protein kinase C; ROS, reactive oxygen species; DFU, diabetic foot ulcer.
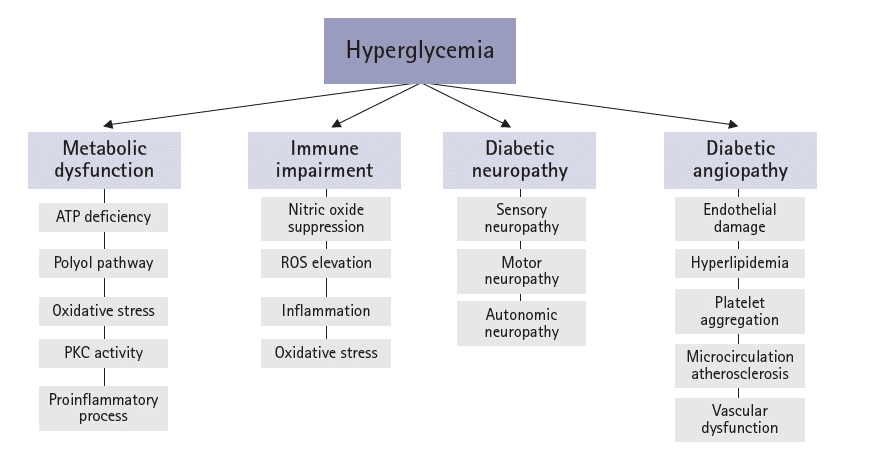
Fig. 3.
The process from callus formation to ulceration formation in patients with diabetes. The interaction of sensory, motor, and autonomic neuropathy can form a callus in the foot. After repeated exposure to external or minor trauma, skin ulcers form through subcutaneous hemorrhages. Adapted from Armstrong et al. [40] with permission of the New England Journal of Medicine.
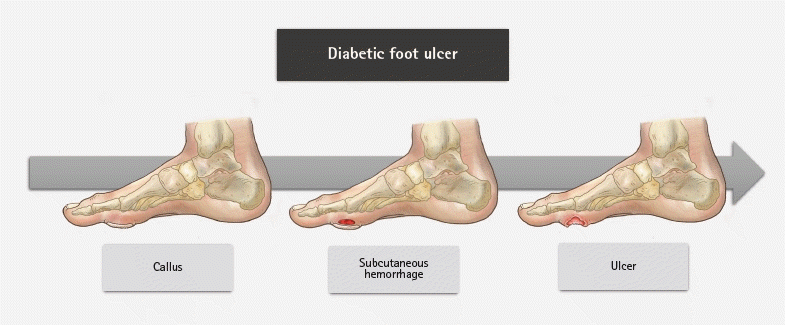
Fig. 4.
Development of diabetic foot ulcers due to diabetic neuropathy. The interplay of metabolic factors, immunopathy, diabetic neuropathy, and diabetic angiopathy fosters the development and progression of infections, ischemic ulcers, or gangrene in individuals with diabetes. Adapted from Schaper et al. [47] with permission from John Wiley & Sons, Ltd.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download