Abstract
Diabetic foot is one of the most devastating consequences of diabetes, resulting in amputation and possibly death. Therefore, early detection and vigorous treatment of infections in patients with diabetic foot are critical. This review seeks to provide guidelines for the therapy and rehabilitation of patients with moderate-to-severe diabetic foot. If a diabetic foot infection is suspected, bacterial cultures should be initially obtained. Numerous imaging studies can be used to identify diabetic foot, and recent research has shown that white blood cell single-photon emission computed tomography/computed tomography has comparable diagnostic specificity and sensitivity to magnetic resonance imaging. Surgery is performed when a diabetic foot ulcer is deep and is accompanied by bone and soft tissue infections. Patients should be taught preoperative rehabilitation before undergoing stressful surgery. During surgical procedures, it is critical to remove all necrotic tissue and drain the inflammatory area. It is critical to treat wounds with suitable dressings after surgery. Wet dressings promote the formation of granulation tissues and new blood vessels. Walking should begin as soon as the patient’s general condition allows it, regardless of the wound status or prior walking capacity. Adequate treatment of comorbidities, including hypertension and dyslipidemia, and smoking cessation are necessary. Additionally, broad-spectrum antibiotics are required to treat diabetic foot infections.
In a narrow sense, diabetic foot refers only to wounds or ulcers on the foot related to diabetes, but the term is generally used as a broader diagnosis that includes various pathological conditions that occur on the foot in patients with diabetes [1]. In Korea, it was reported that the number of patients with diabetes reached 13.2% of the total population in 2021 and exceeded 6 million people. Diabetic foot has a prevalence of 4% to 10% in diabetic patients, with an annual incidence rate of 2.2% to 5.9%, and 25% of diabetic patients develop diabetic foot ulcers (DFUs) during their lifetime [2]. These trends are expected to continue as the diabetic population grows worldwide, resulting in significant medical, social, and economic burdens.
Diabetic foot is a common cause of lower limb amputation, second only to trauma [3]. The 5-year survival rate of amputation for DFU is believed to be less than 50% [4]. Previous research has also found that amputation rates are related to patient clinical outcomes, depending on the severity of the diabetic foot. Ahn [5] reported that approximately 25% of patients with diabetes develop diabetic foot during their lifetime, and 2% experience amputation. Approximately 20% of patients with diabetic foot and moderate or severe infection have undergone partial foot amputation. Previous studies have indicated that the treatment duration and mortality rates increase with the severity of diabetic foot [6]. In addition, recurrence after treatment is common, with approximately 40% of patients reporting recurrence within 1 year and approximately 65% within 5 years [7].
Therefore, early diagnosis and aggressive treatment of infections in patients with diabetic foot are important and require continuous management. In particular, moderate and severe diabetic foot infections may require stringent management and rehabilitation. Although previous studies have reported on the overall management of diabetic foot, there is a lack of specialized reports on moderate and severe infections. Moreover, this article aimed to describe management and rehabilitation guidelines with a focus on moderate and severe diabetic foot.
Patients with diabetes and their families who are at high risk for diabetic foot require broad education about risk factors and adequate management. First, patients who have lost the protective sensation in their feet must be educated on how to compensate for it and detect lesions early [1].
Amputation results in difficulty walking, but recent advances in rehabilitation exercises and prostheses have made it possible to walk more smoothly than in the past. It is important to explain and discuss amputation with the patient to avoid fear that it will prevent them from walking properly.
Several studies have reported a high prevalence of psychiatric disorders, including depressive disorders, in patients with diabetic foot. DFU disturbs the daily lives of patients, altering sleep patterns, impairing mobility, interfering with certain aspects of life such as sexuality, and creating feelings of loneliness, powerlessness, anxiety, and depression [8]. Strong relationships exist between mental health conditions and the increased recurrence of foot ulcers. Therefore, it is important to identify and treat mental health conditions before and after surgery through appropriate psychiatric counseling and education for patients and caregivers [9,10].
The management of diabetic foot requires a multidisciplinary approach that includes not only management of the foot itself but also internal management, such as glycemic and blood pressure control and nutrition, adaptation to daily life through appropriate exercise and rehabilitation, and psychological management, which will be discussed individually below.
Diagnosis begins with history-taking and physical examination. During clinical examination, it is important to check the peripheral pulses of the feet and signs of vascular insufficiency (of which hair loss and muscle atrophy may be indicative) the presence of calluses, and the location of the ulcer. Ulcers are most common in weight-bearing areas, such as the plantar metatarsal head, heel, tips of hammer toes, and other prominent areas (Fig. 1).
Blood tests, such as white blood cell (WBC) count, erythrocyte sedimentation rate, and C-reactive protein level, are commonly requested to aid in diagnosis. However, they are neither sensitive nor specific and are unlikely to be elevated in local or superficial infections [11].
Furthermore, patients with DFUs have higher blood levels of cytokines and chemokines [12]. Interleukin levels are elevated in patients with DFU, along with the development of insulin resistance, abnormal healing, and decreased ulcer healing [13]. Weigelt et al. [12] reported that patients with DFU displayed elevated systemic levels of macrophage inflammatory protein-1 alpha, migration inhibitory factor, and inducible protein-10, decreased RANTES (regulated on activation, normal T cell expressed and secreted) levels.
Bacterial cultures should be obtained if there is a suspicion of infection in a diabetic foot or if there is an exudate (pus). The tissue around the abscess, rather than the abscess itself, should be used as a specimen, which should be obtained as deeply as possible. Wound culture results from a diabetic foot are often polymicrobial, where virulent pathogens (e.g., Staphylococcus aureus or beta-hemolytic streptococci) that are isolated should be treated, and some less virulent isolates (e.g., corynebacteria or coagulase-negative staphylococci) are often contaminants or colonizers that may not need targeted antibiotic treatment. The most common pathogens in diabetic foot are aerobic gram-positive cocci, especially S. aureus, and to a lesser extent, streptococci and coagulase-negative staphylococci [14]. Blood cultures should also be performed if systemic infection is suspected [15].
In addition, imaging studies are necessary to identify areas of infection. In particular, for bone infections, which have a poor prognosis, the gold standard for diagnosing osteomyelitis in diabetic foot is bone biopsy; however, since this procedure is invasive, other imaging tests, such as plain X-rays, magnetic resonance imaging (MRI), bone scans, and WBC single-photon emission computed tomography/computed tomography (SPECT/CT), have been used to identify the infection site in diabetic foot. MRI is the best radiological modality for examining soft tissue abnormalities and may distinguish between soft tissue infections and osteomyelitis. The most reliable diagnostic method is to track the ulcer or sinus tract to the underlying bone and look for a low signal intensity on T1-weighted images, which indicates the presence of marrow edema [16] (Fig. 2). Regarding specificity and diagnostic accuracy, WBC SPECT/CT performs very well for osteomyelitis and soft tissue infections [17]. WBC SPECT/CT effectively reflects diabetic foot osteomyelitis using intensity over a specified threshold. This enables a more precise assessment of areas of elevated WBC intensity, which has been demonstrated to indicate a poor prognosis even when restricted to soft tissue and not in contact with bone [18] (Fig. 3). MRI has previously been utilized as a diagnostic test for diabetic foot osteomyelitis; however, recent investigations have found that WBC SPECT/CT offers similar diagnostic specificity and sensitivity to MRI. Based on bone biopsies for the diagnosis of diabetic osteomyelitis, Sherwood et al. reported that MRI had a sensitivity of 70.6% and a specificity of 40.0%, whereas these values were 82.4% and 73.3%, respectively, for WBC SPECT/CT [19].
Furthermore, ankle-brachial index, transcutaneous oximetry, and CT angiography can be used to assess peripheral blood vessel perfusion in diabetic foot, while monofilament and pin-prick tests can be used to assess peripheral nerve damage (Fig. 4). These tests can assist in evaluating whether a patient requires percutaneous transluminal angioplasty prior to surgery and whether the patient’s clinical outcome can be enhanced with medication [7].
There are several classifications for more systematic diagnosis and treatment. Diabetic foot is categorized into different grades according to international community guidelines. Each classification system for diabetic foot is slightly different but is based on the guidelines published by the International Working Group on the Diabetic Foot in 2019. Most classification systems categorize grade 3 (moderate) as an infection that extends more than 2 cm around the lesion, forms an abscess or gangrene in the deep tissue, or involves muscles, tendons, joints, or bones, while grade 4 (severe) is a systemic infection that includes changes in vital signs such as fever and hypotension [20,21] (Table 1).
Other classification systems exist, such as the Wagner classification system; the Wound, Ischemia, and Foot Infection classification system; and the University of Texas classification system. However, in general, moderate or severe infection refers to infection of deep tissues such as tendons, joints, or bones, or systemic symptoms such as sepsis [22].
Ulcers typically occur in patients with diabetes who have one or more risk factors, such as diabetes-related peripheral neuropathy and/or peripheral artery disease (PAD). Foot deformity, loss of protective sensibility, and reduced joint motion can result in an aberrant biomechanical load on the foot. This causes considerable mechanical stress in specific regions, frequently resulting in skin thickening (calluses). Calluses are often accompanied by subcutaneous hemorrhages and skin ulcers, which further increase the load on the feet. PAD is commonly caused by atherosclerosis, which is present in up to 50% of these patients and is an important risk factor for damaged wound healing, gangrene, and lower extremity amputation [23].
Diabetic foot wounds should be evaluated for size, depth, and placement before appropriate wound dressings are applied. The goal of dressing is to remove debris and necrotic tissue from the wound, absorb exudate, and maintain a reasonable amount of moisture on the wound surface to protect against injury and infection and aid in regeneration. Various dressing formulae are available, and it is critical to select the correct formula for wound care. In superficial ulcers, if there are no significant problems with lower extremity blood flow, methods include closed wound care with gauze, film, hydrocolloid, hydrogel, or foam; dressings with various growth factors, collagen, and other materials; topical drug therapy such as Dermagraft (Organogenesis, Canton, MA, USA); hyperbaric oxygen therapy; and total contact casting [15]. However, as simple wound dressings cannot remove infected necrotic tissue from deeper tissues, surgical intervention is frequently needed to treat full-thickness ulcers or gangrene involving bone [24].
Patients in good systemic condition with the capacity to walk before amputation are very likely to achieve high physical function during rehabilitation after amputation [25]. Patients who have had trouble walking for a long time before amputation, in contrast, will have a longer recovery period and will frequently be unable to restore their walking capacity even after much time and effort. Therefore, walking and activity should be permitted, encouraged, and promoted to the extent that systemic conditions allow [9]. Patients and their caregivers should also be educated and supported in performing rehabilitation exercises [26].
Preoperative physical therapy, sometimes known as ‘pre-rehabilitation,’ is the process of preparing the body to tolerate the stressful, postoperative episodes of inactivity [27]. These pre-rehabilitation programs, which often begin 4 to 6 weeks before surgery, comprise repetitive physical exercises, preferably in conjunction with occupational therapy, psychological assessment, and education. Chest physiotherapy, breathing exercises for lung expansion that may reduce postoperative pneumonia, muscle and joint mobility training, and functional preservation are all part of the preoperative program [28,29].
Widely known surgical treatments are described briefly below. Surgical treatment of DFU is performed when the ulcer is deep and accompanied by bone and soft tissue infections. Surgical treatments are divided into three main types: (1) debridement, (2) reconstruction, and (3) amputation [29]. In diabetic foot infections, it is critical to remove all necrotic tissue and drain the inflammatory material. Amputation may also be considered to remove infected necrotic tissue, which is unlikely to recover [1].
Debridement is used to prevent ulceration of infected or nonviable tissues. If vasculopathy is mild, hyperkeratotic tissue, fibrin, eschar, biofilm, and necrotic tissue should be removed, and drainage should be conducted until healthy, well-bleeding tissue is present. The most popular technique, sharp debridement, involves the removal of necrotic tissue using a knife or scissors. Sharp excisional debridement decreases the bacterial load and stimulates contraction and wound epithelialization [30]. The wound should then be dressed appropriately according to its condition to encourage the development of healthy granulation tissue [31].
The goals of reconstruction are to restore bipedal ambulation, correct skeletal deformities, and prevent ulcerative recurrence. The reconstructive options include skin grafts, local flaps, and free flaps. The flap must have an adequate blood supply, restore sensation while maintaining protective sensation under weight-bearing, and be sturdy enough to withstand the shear pressures experienced while walking [30]. Identifying the patient’s preoperative status is critical prior to surgical intervention. The patient’s status includes comorbid conditions, vascular insufficiency, infection control, and foot deformities. Flap-based reconstruction is generally impossible in patients with severe vascular problems [32].
Amputation is currently the optimal choice when there is evidence of necrosis due to infection or ischemic injury that does not respond to conservative treatment. However, given that patients with diabetes who undergo amputation surgery are significantly less able to perform activities of daily living after amputation and are more likely to die within 5 years, there have been recent attempts to perform limited amputations that spare as much of the heel as possible while performing additional procedures to improve survival [30]. Depending on the location of the amputation, there are several options, including toe, transmetatarsal, Lisfranc, Chopart, Syme, below-knee, and above-knee amputations [9]. A multidisciplinary approach, as well as strict identification of the amputation type and plane, is recommended for effective treatment [26]. Proximal amputation is less likely to result in postoperative wound problems, and patients do not want to undergo repeated anesthesia and surgery. However, amputation is irreversible, and it is important to minimize the area of amputation and consider its function after amputation [15,33].
After surgical treatment, managing the wound using proper dressings is important [27]. Wet dressings promote the formation of granulation tissue and new blood vessels, promoting wound healing. Therefore, various dressing formulations should be used to keep the wound moist; in cases of infection, antibacterial dressing formulations may be used [15,33]. It is important to understand the effectiveness of various dressing products and select one that suits the condition of the wound [5,34] (Table 2).
In negative-pressure wound therapy (NPWT), a wound dressing is applied with constant or intermittent negative pressure to drain the tissue fluid from the area and collect it in a canister [31]. NPWT can be used for chronic wounds with high exudates that have not healed in a long time and have soft tissue defects. This provides negative pressure on the wound area to encourage the production of granulation tissue [15]. However, wound maceration, dressing retention, and wound infection are possible side effects [31].
Patients undergoing amputation frequently have poor gait prior to surgery, and their ambulatory ability deteriorates following surgery. Delirium can occur after surgery, resulting in prolonged recovery time, difficulties with postoperative care, and longer hospital admissions. This impedes the recovery of ambulation [35]. In addition, pulmonary emboli from deep vein thrombosis and pneumonia may occur because of immobilization [36]. Immobilization after amputation can further disrupt ambulatory recovery and create a vicious cycle. Therefore, active gait training following amputation is critical for improving the patient’s overall condition [9].
Strength training should be resumed immediately after surgery to prevent muscle loss and atrophy. Weakness in the lower extremities can affect other joints in the ipsilateral or contralateral lower extremities, causing other problems [9].
Repetitive joint exercises should be performed immediately after surgery because contractures can occur in the remaining joints after amputation. Joint exercises should be performed to avoid equinovarus deformity of the ankle joint and flexion contracture of the knee joint. To prevent equinovarus deformity of the ankle joint, it is necessary to constantly stretch the Achilles tendon and, in severe cases, wear a prosthetic leg to prevent the deformity from persisting [9].
After surgery, if the patient’s general condition allows ambulation should begin as early as possible using a wheelchair, crutches, or other walking aids, regardless of the wound condition and preoperative walking ability. This is essential for maintaining pre-amputation gait and balance; maintaining athletic performance, including cardiorespiratory endurance; and improving joint range of motion and muscle strength [9].
The metabolic control of blood glucose is related to wound healing. In addition, hyperglycemia impairs leukocyte migration and phagocytosis and decreases bactericidal activity. Furthermore, the patient’s systemic nutritional status should be evaluated, and additional nourishment should be administered orally and intravenously if the patient is malnourished [15]. Adequate treatment of comorbidities such as hypertension and dyslipidemia is important, as is smoking cessation [37].
Broad-spectrum antibiotics are essential for the treatment of diabetic foot infections. Multidisciplinary care is recommended because antibiotic selection should be based on various factors, including patient comorbidities, renal function, dialysis status, and community microbial prevalence [22].
Antibiotic selection should be based on the relatively common causal organisms of the infection, regardless of whether the organism has been identified. Common organisms include S. aureus, Streptococcus, Enterobacter cloacae, and Pseudomonas aeruginosa, with Staphylococcus being responsible for high-risk infections that lead to amputation. In contrast, diabetic foot infections are usually mixed infections, and first-line empiric antibiotics include ampicillin-sulbactam, amoxicillin-clavulanic acid, clindamycin, fluoroquinolones, and first-grade cephalosporins [36].
Several principles should be considered during antibiotic therapy. Antibiotics should be used that are efficient against aerobic gram-positive organisms such as S. aureus. As a rule, a narrow-spectrum, first-generation cephalosporin or nafcillin that is effective against gram-positive bacteria is appropriate for mild acute infections. However, in chronic diabetic foot infections that have previously been treated with antibiotics, the possibility of polymicrobial infections, including gram-negative membranous bacteria, should be considered [22]. The duration of treatment in cases with osteomyelitis should be determined by the surgical method and extent of infected residual tissue [22] (Table 3).
The management of diabetic foot requires a multidisciplinary approach that includes not only management of the foot itself but also internal management (such as glycemic and blood pressure control and nutrition), adaptation to daily life through appropriate exercise and rehabilitation, and psychological management.
Diabetic foot is not caused by a single factor, but rather by a combination of factors that contribute to its development and treatment. In diabetic foot with moderate or severe infections, a broad medical understanding and multidisciplinary approach are required, including perioperative management, surgical method selection, patient education, exercise and rehabilitation, metabolism and nutrition, and overall lifestyle modifications. To prevent and treat complications in patients with diabetic feet, a multidisciplinary treatment plan should be developed, and aggressive rehabilitation should be the treatment of choice, along with early medical and surgical management.
Notes
References
2. Bae SY. Treatment of diabetic foot: procedures, surgery, and care. Monthly Diabetes. 2013; 289:26–36.
3. Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007; 4:286–7.

4. Shankar P, Grewal VS, Agrawal S, Nair SV. A study on quality of life among lower limb amputees at a tertiary prosthetic rehabilitation center. Med J Armed Forces India. 2020; 76:89–94.

6. Rubio JA, Jiménez S, Lázaro-Martínez JL. Mortality in patients with diabetic foot ulcers: causes, risk factors, and their association with evolution and severity of ulcer. J Clin Med. 2020; 9:3009.

7. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005; 293:217–28.

8. Ahmad A, Abujbara M, Jaddou H, Younes NA, Ajlouni K. Anxiety and depression among adult patients with diabetic foot: prevalence and associated factors. J Clin Med Res. 2018; 10:411–8.

9. Choi Y. Rehabilitation of patients after diabetic foot amputation. J Korean Med Assoc. 2021; 64:537–42.

10. Shi C, Wang C, Liu H, Li Q, Li R, Zhang Y, et al. Selection of appropriate wound dressing for various wounds. Front Bioeng Biotechnol. 2020; 8:182.

11. Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004; 39(Suppl 2):S83–6.

12. Weigelt C, Rose B, Poschen U, Ziegler D, Friese G, Kempf K, et al. Immune mediators in patients with acute diabetic foot syndrome. Diabetes Care. 2009; 32:1491–6.

13. Wang Y, Shao T, Wang J, Huang X, Deng X, Cao Y, et al. An update on potential biomarkers for diagnosing diabetic foot ulcer at early stage. Biomed Pharmacother. 2021; 133:110991.

14. Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020; 36(Suppl 1):e3280.

16. Erdman WA, Buethe J, Bhore R, Ghayee HK, Thompson C, Maewal P, et al. Indexing severity of diabetic foot infection with 99mTc-WBC SPECT/CT hybrid imaging. Diabetes Care. 2012; 35:1826–31.

17. Lauri C, Glaudemans AW, Campagna G, Keidar Z, Muchnik Kurash M, Georga S, et al. Comparison of white blood cell scintigraphy, FDG PET/CT and MRI in suspected diabetic foot infection: results of a large retrospective multicenter study. J Clin Med. 2020; 9:1645.

18. Low KT, Peh WC. Magnetic resonance imaging of diabetic foot complications. Singapore Med J. 2015; 56:23–33.

19. Sherwood A, Rubitschung K, Killeen A, Crisologo P, Haley R, Hwang H, et al. Comparison of WBC-SPECT/CT and MRI in diagnosis and evaluation of antibiotic response in diabetic foot osteomyelitis. J Nucl Med. 2022; 63(Suppl 2):2772.
20. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA, et al. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020; 36(Suppl 1):e3266.

21. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Fitridge R, Game F, et al. 2023 IWGDF guidelines on the prevention and management of diabetes-related foot disease [Internet]. The International Working Group on the Diabetic Foot (IWGDF); 2023 [cited 2023 Aug 9]. https://iwgdfguidelines.org/wp-content/uploads/2023/07/IWGDF-Guidelines-2023.pdf.
23. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Fitridge R, Game F, et al. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab Res Rev. 2023; e3657.

24. Hong JP, Oh TS. An algorithm for limb salvage for diabetic foot ulcers. Clin Plast Surg. 2012; 39:341–52.

25. Turan Y, Ertugrul BM, Lipsky BA, Bayraktar K. Does physical therapy and rehabilitation improve outcomes for diabetic foot ulcers? World J Exp Med. 2015; 5:130–9.

26. Uustal H. Prosthetic rehabilitation issues in the diabetic and dysvascular amputee. Phys Med Rehabil Clin N Am. 2009; 20:689–703.

27. Ditmyer MM, Topp R, Pifer M. Prehabilitation in preparation for orthopaedic surgery. Orthop Nurs. 2002; 21:43–51.

28. Hijmans JM, Dekker R, Geertzen JH. Pre-operative rehabilitation in lower-limb amputation patients and its effect on post-operative outcomes. Med Hypotheses. 2020; 143:110134.

29. Castillo R, Haas A. Chest physical therapy: comparative efficacy of preoperative and postoperative in the elderly. Arch Phys Med Rehabil. 1985; 66:376–9.
31. Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007; 13:30–9.

32. Oh TS, Lee HS, Hong JP. Diabetic foot reconstruction using free flaps increases 5-year-survival rate. J Plast Reconstr Aesthet Surg. 2013; 66:243–50.

34. Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: current advances and future directions. J App Polym Sci. 2019; 136:47738.

35. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020; 125:492–504.

Fig. 1.
Clinical photos of diabetic foot ulcer. (A) Lateral aspect. (B) Dorsal aspect. (C) Volar aspect.
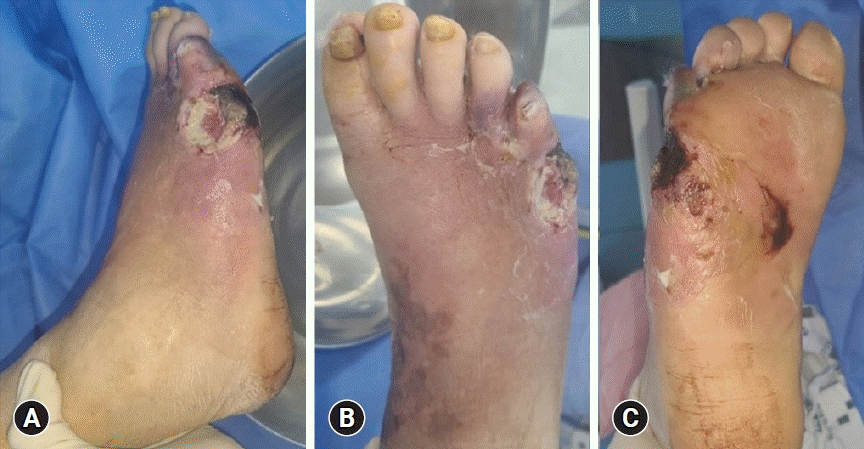
Fig. 2.
Magnetic resonance imaging (MRI) of diabetic foot ulcers shows bone marrow edema, diffuse soft tissue edema, and cortical irregularity. (A) Axial T1-weighted MRI shows ill-defined heterogeneous low signal intensity change with cortical destruction in the right 4th toe. (B) Axial T2-weighted fat-suppressed MRI shows high signal intensity in the proximal, middle, and distal phalanx of the right 4th toe which may indicate osteomyelitis.
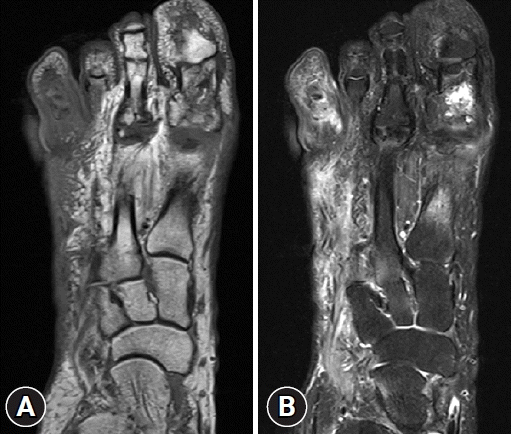
Fig. 3.
White blood cell single-photon emission computed tomography/computed tomography of diabetic ulcer demonstrates focal abnormal leukocyte accumulations in the right 5th toe which indicates active infection or inflammation with suspicion of bone involvement in the proximal phalanx.
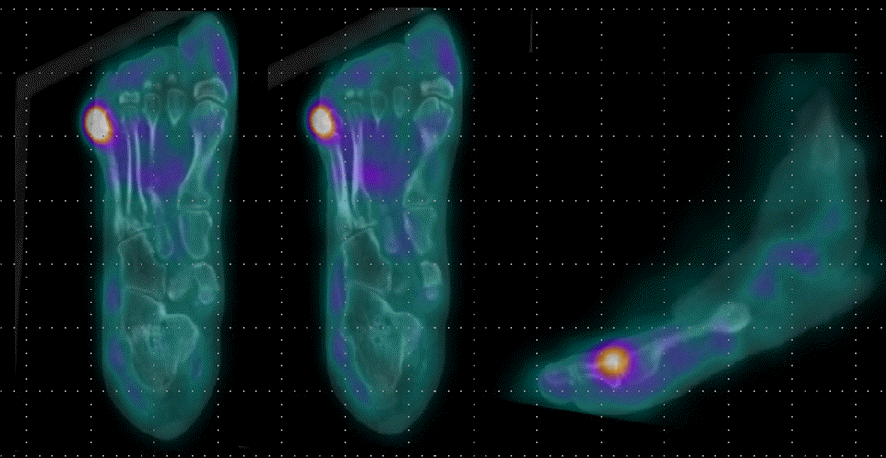
Fig. 4.
Computed tomography angiography shows diffuse atherosclerosis with multifocal ulcers on aortoiliac and lower extremity arteries and near occlusion on the anterior tibial artery and posterior tibial artery.
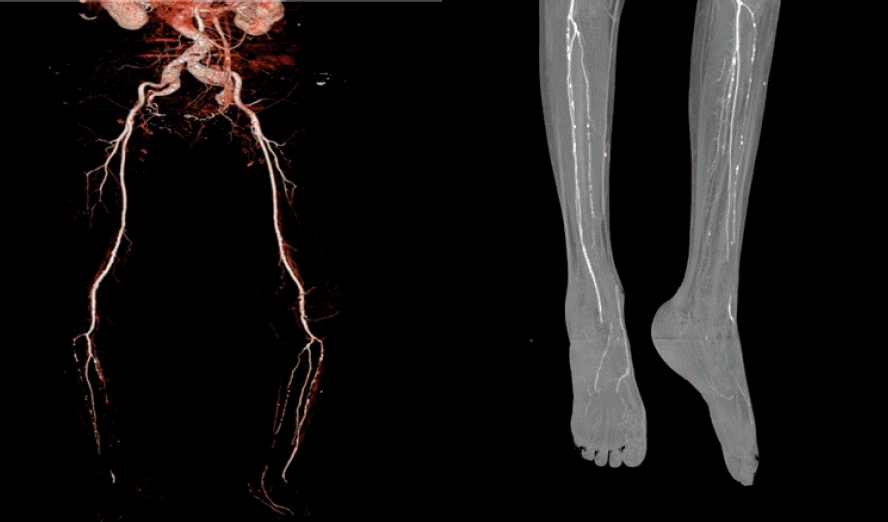
Table 1.
Guidelines of International Working Group on the Diabetic Foot/Infectious Diseases Society of America system
PEDIS, perfusion, extent, depth, infection, and sensation.
Adapted from 2023 International Working Group on the Diabetic Foot guidelines [21].
Table 2.
Characteristics and applications of the dressing agents
Adapted from Rezvani Ghomi et al. [34] according to the Creative Commons License.
Table 3.
Postoperative management including metabolic and lifestyle adjustments and antibiotic treatment




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download