Abstract
Background
The aim of this study was to evaluate the risk factors for prostate-specific antigen (PSA) persistence in pathological stage T3aN0 prostate cancer (PCa) after robot-assisted laparoscopic radical prostatectomy (RALP).
Methods
A retrospective study was performed on 326 patients with pT3aN0 PCa who underwent RALP between March 2020 and February 2022. PSA persistence was defined as nadir PSA of >0.1 ng/mL after RALP, and the risk factors for PSA persistence were evaluated using logistic regression analysis.
Results
Among 326 patients, 61 (18.71%) had PSA persistence and 265 (81.29%) had PSA of <0.1 ng/mL after RALP (successful radical prostatectomy [RP] group). In the PSA persistence group, 51 patients (83.61%) received adjuvant treatment. Biochemical recurrence occurred in 27 patients (10.19%) in the successful RP group during the mean follow-up period of 15.22 months. Multivariate analysis showed that the risk factors for PSA persistence were large prostate volume (hazard ratio [HR], 1.017; 95% confidence interval [CI], 1.002–1.036; p=0.046), lymphovascular invasion (LVI) (HR, 2.605; 95% CI, 1.022–6.643; p=0.045), and surgical margin involvement (HR, 2.220; 95% CI, 1.110–4.438; p=0.024).
Recently, active surveillance has been recommended as a treatment for early prostate cancer (PCa), owing to the increasing early detection rate of PCa. The development of diagnostic tools for PCa, such as prostate-specific antigen (PSA), prostate health index, and prostate multiparametric magnetic resonance imaging (mpMRI), has led to increased detection of PCa at an early stage [1,2]. However, approximately 10% of patients with PCa are initially diagnosed at an advanced stage [3]. Unlike localized PCa, advanced PCa is a life-threatening condition requiring multimodal treatment [4].
Previously, radical prostatectomy (RP) was not performed for advanced PCa, and palliative treatments such as androgen deprivation therapy (ADT) or radiation therapy were performed in most cases. However, the effectiveness of RP as a treatment for advanced PCa has only recently been reported [5]. Many reports have been published on the effectiveness of RP for the treatment of locally advanced T3 PCa [6,7]. Koo et al. [8] reported that treating advanced PCa with RP produced a lower cancer-specific mortality rate than with radiation and ADT combined treatment. Recently, RP has been suggested as an initial treatment option for advanced PCa [9].
Although biochemical recurrence (BCR) may occur, the efficacy of robot-assisted laparoscopic radical prostatectomy (RALP) in patients with T3aN0 PCa can be defined as successful RP when the PSA drops below 0.1 ng/mL after surgery. Adjuvant or early salvage radiotherapy is recommended for persistent PSA. Although there is no clear definition of PSA persistence, it is generally defined as PSA of >0.1 ng/mL at 6 to 8 weeks after RP. However, there are many cases where the nadir PSA value is lowered even without clinically specific treatment.
Therefore, in this study, the characteristics of patients with persistent PSA and the risk factors for PSA persistence were evaluated. Early adjuvant or salvage treatment may be determined through patient selection.
Ethical statements: This study was performed in accordance with applicable laws and regulations, good clinical practice, and ethical principles, as described in the Declaration of Helsinki. The Institutional Review Board (IRB) of Samsung Medical Center approved this study (IRB No: 2022-08-002). The IRB waived the requirement for informed patient consent owing to the retrospective nature of this study. Registered patient information was extracted only from Samsung Medical Center, Seoul, Korea. All data were analyzed after anonymization and were collected every month.
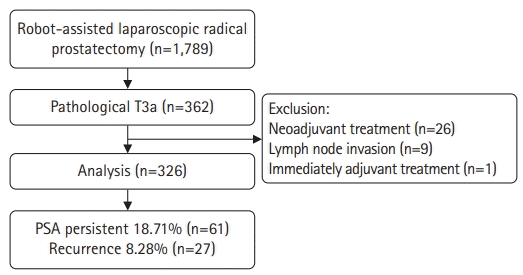
We performed a retrospective analysis of 362 patients with pathological stage T3a PCa among 1,789 patients who underwent RALP between March 2020 and February 2022. Among the 362 patients, those who received neoadjuvant treatment, those with confirmed lymph node involvement, and those who received adjuvant treatment without PSA follow-up after surgery were excluded from the analysis.
PSA persistence was defined as a nadir PSA level of >0.1 ng/mL after RALP. BCR was defined as a case in which the PSA level was <0.1 ng/mL and then ≥0.2 ng/mL twice consecutively during follow-up. Furthermore, a nadir PSA level after RP of <0.1 ng/mL defined a successful RP group.
All the patients underwent mpMRI, computed tomography, and whole-body bone scanning before RALP. To evaluate the patients' baseline characteristics, age, body mass index, serum PSA levels, prostate volume (measured by transrectal ultrasonography or magnetic resonance imaging), PSA density, results of preoperative biopsy (including Gleason score, positive core percentage, and highest tumor volume percentage in core), and clinical stage were evaluated. Peri- and post-operative outcomes, including operative time, estimated blood loss, pathological outcomes, pathological stages, nadir PSA value, adjuvant treatment, and follow-up period, were also assessed.
A 4-Arm da Vinci Robotic System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was used for the surgery, and the transabdominal approach was performed using six ports. A 12-mm camera port, three 8-mm robot ports, and an additional two 12-mm assist ports were used above the umbilicus. The surgery was performed according to the general surgical method. The physician decided whether lymph node (LN) dissection and neurovascular bundle (NVB) preservation were performed. LN dissection was performed in the pelvic cavity. Seven experienced urologists performed the surgeries.
In this study, Student t-tests were used to compare continuous variables, and the chi-square tests were used to compare categorical variables. Risk factors for PSA persistence were analyzed using logistic regression analysis. IBM SPSS ver. 21.0 for Windows (IBM Corp., Armonk, NY, USA) was used as a statistical analysis program, and p-values of <0.05 were considered statistically significant.
Among the 362 patients with pT3aN0 PCa, the final analysis was performed on 326 of them, excluding 26 patients who received neoadjuvant treatment, nine with confirmed LN invasion, and one who received immediate adjuvant treatment without follow-up after RALP.
Among the 326 patients, 61 (18.71%) had PSA persistence and 265 (81.29%) had PSA of <0.1 ng/mL after RALP (successful RP group). In the PSA persistence group, 51 patients (83.61%) received adjuvant treatment. BCR occurred in 27 patients (10.19%) in the successful RP group during the mean follow-up period of 15.22 months (Fig. 1).
The mean age of the group in which the nadir PSA was <0.1 ng/mL (successful RP group) was 67.20±6.81 years, and the mean age of the PSA persistence group was 67.75±6.77 years (p=0.569). The mean preoperative PSA of the successful RP group was 11.48±12.17 ng/mL, and that for the PSA persistence group was 19.37±13.90 ng/mL (p<0.001). The prostate volume of the successful RP group was 30.51±15.25 mL, and that of the PSA persistence group was 36.23±19.83 mL (p=0.015). Furthermore, significant differences were confirmed in the International Society of Urological Pathology grade, clinical T stage, proportion of positive cores among biopsy cores, Prostate Imaging Reporting & Data System, and size of the index tumor between the two groups (p<0.05) (Table 1).
Regarding surgical outcomes, bilateral NVB sparing was frequently performed in the successful RP group (31.70% vs. 9.84%) (p<0.001). Lymphovascular invasion (LVI) was 7.93% and 19.67% in the successful RP and PSA persistence groups, respectively (p=0.006). The proportion of cancer volume in the total prostate volume was 21.98%±15.48% in the successful RP group and 32.20%±21.42% in the PSA persistence group (p<0.001). The surgical margin involvement rate was 30.57% in the successful RP group and 50.82% in the PSA persistence group (p=0.003). Adjuvant or salvage treatment was performed in 7.55% of the patients in the successful RP group and 83.61% of those in the persistent PSA group (p<0.001). In 10.19% of patients in the successful RP group, BCR occurred during follow-up (Table 2).
The risk factors for PSA persistence were large prostate volume (hazard ratio [HR], 1.017; 95% confidence interval [CI], 1.002–1.036; p=0.046), LVI (HR, 2.605; 95% CI, 1.022–6.643; p=0.045), and surgical margin involvement (HR, 2.220; 95% CI, 1.110–4.438; p=0.024) (Table 3).
In this study, we evaluated the risk factors of PSA persistence after RALP in patients with pT3aN0 PCa. The statistically significant factors were large prostate volume, LVI, and surgical margin involvement. This result can be used to help decide post-RALP management for patients with pT3aN0 PCa.
Kliment et al. [10] reported a 5-year PCa-specific survival rate of 98% and a 10-year survival rate of 76.3% after retroperitoneal RP in patients with T3b–T4 and N0-1 PCa. It was concluded that RP is an effective option for multimodal treatments of advanced PCa. Casey et al. [11] reported that RALP showed favorable treatment outcomes in 71% of patients with T3 or higher PCa. In addition, there are few reports on the efficacy of RP for locally advanced PCa, but it has been suggested as a relatively successful treatment option [9]. In this study, although BCR occurred in 10.19% of patients, 81.29% of patients with pT3N0 disease were evaluated as having successful RP.
Hajili et al. [12] reported that 82% of patients who received neoadjuvant ADT and RP for T4 PCa had a survival duration of 150 months. However, in their study, the final stage was confirmed to be T2–3 in 95.7% of the patients. Most previous studies were performed based on the clinical stage. The present study could have clinical significance because the evaluation was performed with T3aN0 as the final pathological stage. However, the PSA persistence group had a higher clinical stage than the non-PSA persistence group. Because a pathologic review was not performed, the degree of capsular invasion, whether focal or extensive, may have affected the clinical staging. Extensive capsular invasion may be associated with an advanced clinical stage and PSA persistence, and further evaluation should be performed.
PSA persistence after RP has been defined in past studies by several criteria, such as a PSA level of 0.03, 0.1, or 0.5 ng/mL [13-17]. This study defined PSA persistence after RP as PSA of >0.1 ng/mL, and 83.61% of the patients with PSA persistence received adjuvant or salvage treatment. The median follow-up period of the remaining 10 patients (16.39%) was relatively short (12.0 months), and additional treatment would be required in the future. It would be reasonable to define PSA persistence as >0.1 ng/mL.
PSA persistence is known to increase the risk of metastasis and adversely affect cancer-specific survival [18,19]. However, in previous studies, PSA persistence was diagnosed 6 to 8 weeks after RP. In the present study, PSA persistence was defined by the nadir PSA value, not the PSA value at a specific time point after RALP, and the time to nadir PSA was a median of 11.86 weeks after RALP. In addition, among the patients in the successful RP group, 45 out of 245 who did not receive adjuvant or salvage treatment (18.36%) had PSA levels of ≥0.1 ng/mL at 4 to 6 weeks after RP. As a result, the design of this study may help determine adjuvant or early salvage treatment in real clinical practice rather than using PSA persistence at a single time point after RP.
In our results, a larger prostate size affected PSA persistence. However, in previous studies, smaller prostate size was reported as a risk factor for poor progression after RP [20,21]. A large prostate is highly likely to be a remnant of benign prostate tissue after RP, and the possibility that this caused persistent PSA could not be excluded. In addition, most patients with PSA persistence (83.61%) received adjuvant treatment, and there is a possibility of overtreatment in the case of remnant benign prostate tissue. A clear mechanism between prostate size and PCa prognosis has not yet been elucidated, and our findings need to be confirmed through a large-scale study in the future.
In addition, pathological results showed that LVI and surgical margin involvement independently affected PSA persistence. LVI is an adverse pathologic feature, with an incidence between 5.1% and 46.3% in patients with RP [22]. LVI is associated with a higher PSA level, higher Gleason score, more advanced stage, higher rate of LN involvement, and higher risk of BCR [23]. Recently, Jamil et al. [24] reported that LVI affected overall survival in T3a or higher PCa, similar to the results of this study.
Moreover, Zhang et al. [25] reported surgical margin involvement as a poor prognostic factor after RP in a systematic review. Hegemann et al. [26] reported that approximately 75% of patients with pT3a PCa with a positive surgical margin after RP required adjuvant treatment such as radiotherapy or ADT. In addition, among these patients, 24.46% had persistent PSA, and adjuvant treatment was performed in 91.30% of them. In the present study, adjuvant treatment was performed in 28 of 31 patients (90.32%) with margin involvement in the PSA persistence group. However, only 11 of 81 patients (13.58%) with margin involvement in the successful RP group underwent adjuvant treatment. Adjuvant treatment may be required when LVI or marginal involvement is accompanied by PSA persistence.
Our results from this study suggest that after RP in patients with pT3aN0 PCa, if large prostate size, LVI, or surgical margin involvement is present, PSA persistence can continue even after follow-up, and early additional treatment could improve prognosis. This study is a retrospective study, and its limitations are the relatively small number of patients enrolled and the short follow-up period. Furthermore, in most cases of PSA persistence, adjuvant treatment was performed; therefore, it was not possible to evaluate BCR in this group. Quantitative analysis of prostate capsular invasion was also impossible. There may have also been confounding bias in this study. Seven surgeons performed the surgery using different apical or bladder neck dissection techniques during RP. To draw concrete conclusions, a large-scale multicenter study needs to be performed in the future.
In patients with pT3aN0 disease after RALP, large prostate size, LVI, and surgical margin involvement are risk factors for PSA persistence. These patients may require adjuvant treatment for a better prognosis.
References
1. Rakauskas A, Tawadros T, Lucca I, Herrera F, Bourhis J, Burruni R, et al. Active surveillance in males with low- to intermediate-risk localized prostate cancer: a modern prospective cohort study. Investig Clin Urol. 2021; 62:416–22.

4. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017; 71:618–29.

5. Jang TL, Patel N, Faiena I, Radadia KD, Moore DF, Elsamra SE, et al. Comparative effectiveness of radical prostatectomy with adjuvant radiotherapy versus radiotherapy plus androgen deprivation therapy for men with advanced prostate cancer. Cancer. 2018; 124:4010–22.

6. van den Ouden D, Hop WC, Schröder FH. Progression in and survival of patients with locally advanced prostate cancer (T3) treated with radical prostatectomy as monotherapy. J Urol. 1998; 160:1392–7.

7. Gözen AS, Akin Y, Ates M, Hruza M, Rassweiler J. Impact of laparoscopic radical prostatectomy on clinical T3 prostate cancer: experience of a single centre with long-term follow-up. BJU Int. 2015; 116:102–8.

8. Koo KC, Cho JS, Bang WJ, Lee SH, Cho SY, Kim SI, et al. Cancer-specific mortality among Korean men with localized or locally advanced prostate cancer treated with radical prostatectomy versus radiotherapy: a multi-center study using propensity scoring and competing risk regression analyses. Cancer Res Treat. 2018; 50:129–37.

9. Moris L, Cumberbatch MG, Van den Broeck T, Gandaglia G, Fossati N, Kelly B, et al. Benefits and risks of primary treatments for high-risk localized and locally advanced prostate cancer: an international multidisciplinary systematic review. Eur Urol. 2020; 77:614–27.
10. Kliment J Jr, Elias B, Baluchova K, Kliment J Sr. The long-term outcomes of radical prostatectomy for very high-risk prostate cancer pT3b-T4 N0-1 on definitive histopathology. Cent European J Urol. 2017; 70:13–9.
11. Casey JT, Meeks JJ, Greco KA, Wu SD, Nadler RB. Outcomes of locally advanced (T3 or greater) prostate cancer in men undergoing robot-assisted laparoscopic prostatectomy. J Endourol. 2009; 23:1519–22.

12. Hajili T, Ohlmann CH, Linxweiler J, Niklas C, Janssen M, Siemer S, et al. Radical prostatectomy in T4 prostate cancer after inductive androgen deprivation: results of a single-institution series with long-term follow-up. BJU Int. 2019; 123:58–64.

13. Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017; 376:417–28.

14. Wiegel T, Bartkowiak D, Bottke D, Thamm R, Hinke A, Stöckle M, et al. Prostate-specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse-free survival and overall survival: 10-year data of the ARO 96-02 trial. Int J Radiat Oncol Biol Phys. 2015; 91:288–94.
15. Rogers CG, Khan MA, Craig Miller M, Veltri RW, Partin AW. Natural history of disease progression in patients who fail to achieve an undetectable prostate-specific antigen level after undergoing radical prostatectomy. Cancer. 2004; 101:2549–56.

16. Moreira DM, Presti JC Jr, Aronson WJ, Terris MK, Kane CJ, Amling CL, et al. Natural history of persistently elevated prostate specific antigen after radical prostatectomy: results from the SEARCH database. J Urol. 2009; 182:2250–5.

17. Gandaglia G, Boorjian SA, Parker WP, Zaffuto E, Fossati N, Bandini M, et al. Impact of postoperative radiotherapy in men with persistently elevated prostate-specific antigen after radical prostatectomy for prostate cancer: a long-term survival analysis. Eur Urol. 2017; 72:910–7.

18. Fossati N, Karnes RJ, Colicchia M, Boorjian SA, Bossi A, Seisen T, et al. Impact of early salvage radiation therapy in patients with persistently elevated or rising prostate-specific antigen after radical prostatectomy. Eur Urol. 2018; 73:436–44.

19. Preisser F, Chun FK, Pompe RS, Heinze A, Salomon G, Graefen M, et al. Persistent prostate-specific antigen after radical prostatectomy and its impact on oncologic outcomes. Eur Urol. 2019; 76:106–14.

20. Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005; 23:7546–54.

21. Min SH, Park YH, Lee SB, Ku JH, Kwak C, Kim HH. Impact of prostate size on pathologic outcomes and prognosis after radical prostatectomy. Korean J Urol. 2012; 53:463–6.

22. Loeb S, Roehl KA, Yu X, Antenor JA, Han M, Gashti SN, et al. Lymphovascular invasion in radical prostatectomy specimens: prediction of adverse pathologic features and biochemical progression. Urology. 2006; 68:99–103.

23. May M, Kaufmann O, Hammermann F, Loy V, Siegsmund M. Prognostic impact of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2007; 99:539–44.

24. Jamil M, Rakic N, Sood A, Keeley J, Modonutti D, Novara G, et al. Impact of lymphovascular invasion on overall survival in patients with prostate cancer following radical prostatectomy: stage-per-stage analysis. Clin Genitourin Cancer. 2021; 19:e319–25.

Table 1.
Baseline characteristics
| Characteristic | PSA ≤0.1 ng/mL | PSA persistent | p-value |
|---|---|---|---|
| No. of patients | 265 | 61 | |
| Age (yr) | 67.20±6.81 | 67.75±6.77 | 0.569 |
| Body mass index (kg/m2) | 25.11±2.62 | 25.34±2.49 | 0.521 |
| Anti-coagulant | 86 (32.45) | 16 (26.23) | 0.345a) |
| ASA classification | |||
| I | 31 (11.70) | 3 (4.92) | 0.436a) |
| II | 187 (70.57) | 46 (75.41) | |
| III, IV | 47 (17.73) | 12 (19.67) | |
| Smoking | |||
| Smoker | 24 (9.06) | 5 (8.20) | 0.255a) |
| Ex-smoker | 153 (57.74) | 42 (68.85) | |
| 5-Alpha reductase inhibitors | 31 (11.70) | 2 (3.28) | 0.049a) |
| Familial history | 19 (7.17) | 1 (1.64) | 0.105a) |
| IIEF-5 | 10.83±7.04 | 10.32±7.30 | 0.621 |
| Erectile function domain | 11.56±9.72 | 10.51±9.36 | 0.442 |
| PSA (ng/mL) | 11.48±12.17 | 19.37±13.90 | <0.001 |
| Prostate volume (mL) | 30.51±15.25 | 36.23±19.83 | 0.015 |
| PSA density (ng/mL2) | 0.39±0.32 | 0.62±0.46 | <0.001 |
| No. of previous biopsy | 0.17±0.51 | 0.16±0.52 | 0.977 |
| Clinical T stage | |||
| T1–T2 | 114 (43.02) | 15 (24.59) | 0.001a) |
| T3a | 140 (52.83) | 36 (59.02) | |
| T3b–T4 | 11 (4.15) | 10 (16.39) | |
| ISUP grade | |||
| 1 | 33 (12.45) | 3 (4.92) | 0.008a) |
| 2 | 77 (29.06) | 9 (14.75) | |
| 3 | 73 (27.55) | 21 (34.43) | |
| 4 | 61 (23.02) | 25 (40.98) | |
| 5 | 21 (7.93) | 3 (4.92) | |
| Positive cores (%) | 48.11±24.40 | 56.04±25.52 | 0.032 |
| Highest tumor rate in core (%) | 62.57±27.06 | 66.85±26.07 | 0.254 |
| PI-RADS of index lesion | |||
| 2 | 6 (2.26) | 1 (1.64) | 0.033a) |
| 3 | 6 (2.26) | 1 (1.64) | |
| 4 | 92 (34.72) | 10 (16.39) | |
| 5 | 155 (58.49) | 48 (78.69) | |
| Size of index lesion (cm) | 1.74±1.03 | 2.20±0.84 | 0.001 |
| No. of PI-RADS 3 to 5 | 1.39±0.71 | 1.26±0.63 | 0.194 |
Values are presented as number only, mean±standard deviation, or number (%).
PSA, prostate-specific antigen; ASA, American Society of Anesthesiologists; IIEF, International Index of Erectile Function; ISUP, International Society of Urological Pathology; PI-RADS, Prostate Imaging Reporting & Data System.
The p-value was analyzed by the Student t-test or
Table 2.
Surgical and oncological outcomes
| Variable | PSA ≤0.1 ng/mL | PSA persistent | p-value |
|---|---|---|---|
| No. of patients | 265 | 61 | |
| Operation time (min) | 162.34±40.46 | 161.92±43.40 | 0.945 |
| Estimated blood loss (mL) | 163.92±85.27 | 163.11±101.51 | 0.954 |
| Lymph node dissection | |||
| Unilateral | 20 (7.55) | 5 (8.20) | <0.001a) |
| Bilateral | 22 (8.30) | 16 (26.23) | |
| NVB sparing | |||
| Unilateral | 116 (43.77) | 27 (44.26) | <0.001a) |
| Bilateral | 84 (31.70) | 6 (9.84) | |
| ISUP grade | |||
| 1 | 1 (0.38) | 0 (0) | 0.001a) |
| 2 | 107 (40.38) | 10 (16.39) | |
| 3 | 97 (36.60) | 22 (36.07) | |
| 4 | 28 (10.57) | 13 (21.31) | |
| 5 | 32 (12.08) | 16 (26.23) | |
| Perineural invasion | 256 (96.60) | 60 (98.36) | 0.473a) |
| Lymphovascular invasion | 21 (7.93) | 12 (19.67) | 0.006a) |
| Multifocality | 135 (50.94) | 28 (45.90) | 0.445a) |
| Tumor volume (%) | 21.98±15.48 | 32.20±21.42 | <0.001 |
| Margin involvement | 81 (30.57) | 31 (50.82) | 0.003a) |
| Biochemical recurrence | 27 (10.19) | NA | |
| Adjuvant or salvage treatment | 20 (7.55) | 51 (83.61) | <0.001a) |
| Follow-up period (mo) | 15.22±7.02 | 16.25±7.18 | 0.313 |
Table 3.
Logistic regression analysis for prostate-specific antigen persistent in pT3a prostate cancer




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download