Abstract
Acute phlegmonous esophagitis (APE) is a rare and fatal disease. Phlegmonous infection involves the submucosal layer and muscularis propria but not the mucosal layer. Because surgery is not the first treatment option for this disease, an accurate diagnosis is crucial. Herein, we report three cases of APE with various clinical features. All patients were successfully treated with antibiotics and appropriate medical procedures.
Although acute phlegmonous esophagitis (APE) is very rare, it is life-threatening. Its most important feature is the spread of infection to the submucosa rather than the mucosa [1]. Early diagnosis of APE is important because the first treatment option for this disease is not a surgical approach. Herein, we report three cases of APE with various clinical features.
Ethical statements: This study was exempted from review by the Institutional Review Board (IRB) of Daegu Catholic University Medical Center (IRB No: CR-23-012). The need for informed consent was waived owing to the retrospective nature of the study and patient data anonymization.
A 30-year-old man who complained of left neck tenderness, sore throat, and odynophagia for 3 days was admitted to the Department of Ear, Nose & Throat at Daegu Catholic University Medical Center. He had no specific medical history, except for uncontrolled type 2 diabetes mellitus (DM). His body temperature and pulse rate were 39.3°C and 100 beats/min, respectively, and blood pressure and respiration rate were normal. Laboratory findings revealed a leukocyte count of 10,300/µL, C-reactive protein (CRP) level of 152.8 mg/L, serum glucose level of 293 mg/dL, and increased glycated hemoglobin level of 13.5% (ranges: leukocyte count, 3,600–9,600/µL; CRP, <5.0 mg /L; serum glucose, 74–109 mg/dL; glycated hemoglobin, 4.4%–6.3%). Physical examination showed swelling and erythema with tenderness in the left neck, which was considered a neck infection. Neck computed tomography (CT) and chest CT revealed thickening with intramural low density of the entire esophageal wall and multiple air bubbles. However, no evidence of pleural effusion, mediastinal fluid collection, or gas formation was observed (Fig. 1A). The stomach wall of the patient was unremarkable, except for mild thickening. Esophagogastroduodenoscopy (EGD) revealed edematous esophageal mucosa, an opening assumed to be pus drainage immediately below the upper esophageal sphincter, and another opening near the cardia (Fig. 1B). These findings indicated a diagnosis of phlegmonous esophagitis with neck infection, and Klebsiella pneumonia was observed in the blood cultures of the patient. The patient was transferred to the Department of Chest Surgery on hospital day (HD) 2. The patient was treated with intravenous antibiotics (amoxicillin/sulbactam, isepamicin, and clindamycin) and total parenteral nutrition (TPN) via a central venous catheter. Nil per os (NPO) was maintained to avoid injury and facilitate esophageal healing. The general condition of the patient, inflammation, and laboratory findings started to improve following strict control of DM. On HD 5, chest CT revealed improvements in esophageal wall thickening, submucosal air, and fluid collection (Fig. 1C). On HD 9, the hospitalization duration was expected to be extended; thus, to ensure sufficient nutritional support, enteral feeding was initiated through a nasojejunal tube. On HD 24, chest CT revealed a considerable decrease in air bubbles along the esophagus. On HD 46, EGD revealed a normal mucosal appearance with a smaller and cleaner opening than observed previously (Fig. 1D). An esophagogram showed no passage disturbance or leakage (Fig. 1E). The patient was started on a restricted oral diet and was discharged on HD 52 without complications.
A 40-year-old man presented to the emergency room with chest pain that had occurred 3 days earlier. His medical history included type 2 DM and chronic alcoholism. The patient complained of black stools that had occurred for 2 months. Following admission, he vomited greenish bile and blood twice. His vital signs were stable, and the laboratory findings revealed an elevated leukocyte count of 14,000/µL, CRP level of 41.2 mg/L, procalcitonin level of 0.84 µg/L, and lactate level of 5.1 mmol/L (ranges: leukocyte count, 3,600–9,600/µL; CRP, <5.0 mg/L; procalcitonin, <0.5 mg/mL; lactate, 0.7–2.1 mmol/L) and normal hemoglobin level of 13.6 g/dL (range, 12.9–16.9 g/dL). An initial chest CT revealed only a thickened esophageal wall (Fig. 2A). An initial EGD revealed only diffuse edematous mucosa in the distal esophagus (Fig. 2B). The patient was started on antibiotics (ceftriaxone and metronidazole) for suspected esophagitis. On HD 2, chest X-ray imaging showed bilateral lung field haziness, and the patient complained of tachycardia, squeezing chest pain, and dyspnea. The chest CT revealed bilateral pleural fluid and esophageal wall thickening (Fig. 2A). The haziness on the chest X-ray image worsened, and fever developed. The antibiotics were changed to piperacillin/tazobactam, and a pigtail catheter was inserted in the right thoracic cavity for drainage. Through this catheter, 950 mL of exudate was drained, which was positive for K. pneumoniae on culture test. On HD 7, NPO was stopped, and the patient was allowed to take sips of water. On HD 10, a soft diet was initiated, as there were no specific abnormal findings on follow-up EGD (Fig. 2D). The patient’s general condition and CRP level improved; however, his leukocyte count and absolute neutrophil count (ANC) were as low as 2,300/µL and 100/µL, respectively (ranges: leukocyte count, 3,600–9,600/µL; ANC, 1,800–7,700/µL), and his fever persisted. The antibiotics were switched to levofloxacin on suspicion of drug-induced leukopenia. On HD 17, the pigtail catheter was removed, the ANC recovered, fever subsided. On HD 18, the chest CT revealed improved esophageal wall edema and submucosal fluid collection (Fig. 2C) and the patient was discharged.
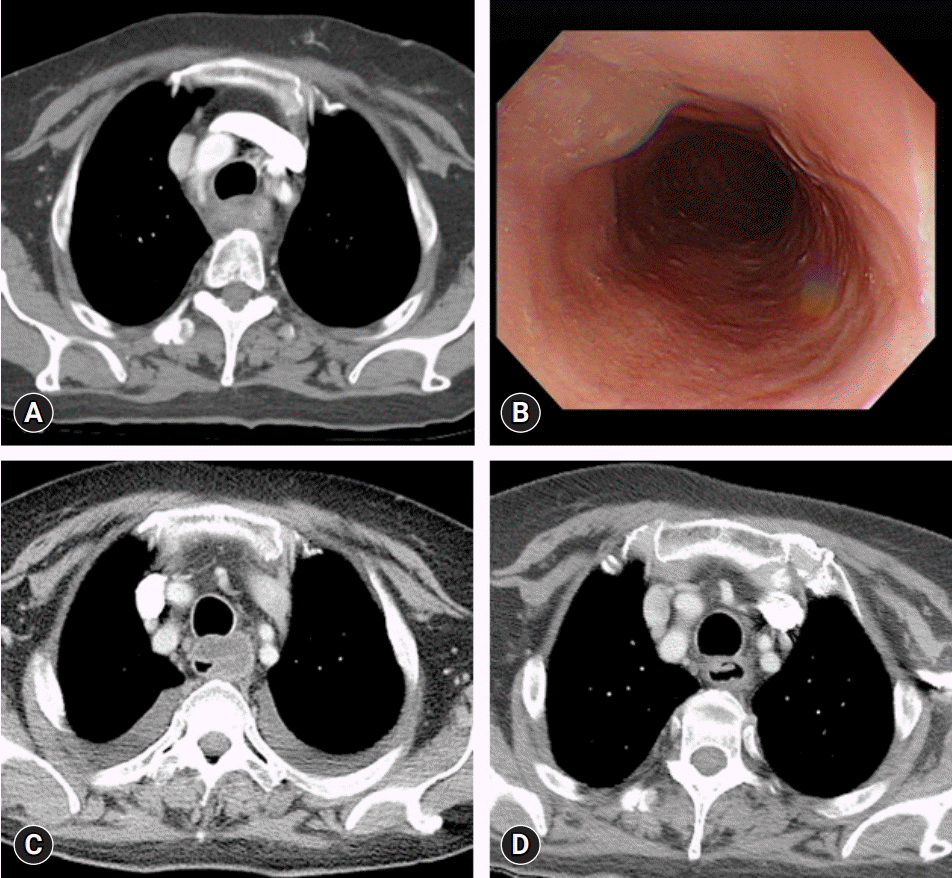
A 67-year-old woman with exacerbated and persistent chest pain since the previous day was transferred to the emergency room. She had been treated for acute pyelonephritis 3 months previously and had type 2 DM. Her vital signs were normal, except for a fever of 38.5°C. The patient was drowsy and confused. The laboratory findings indicated an elevated leukocyte count of 13,900/µL, CRP level of 12.2 mg/L, and glucose level of 603 mg/dL (ranges: leukocyte count, 3,600–9,600/µL; CRP, <5.0 mg/L; serum glucose, 74–109 mg/dL). The patient’s mental status improved following glucose control and hydration. Blood and sputum culture results of the patient were negative. Initial chest CT revealed diffuse edematous wall thickening in the cervical and upper thoracic esophagus (Fig. 3A). Intravenous antibiotics (ceftriaxone and metronidazole) were administered for suspected esophagitis. An EGD showed edematous esophageal wall (Fig. 3B). On HD 5, the fever persisted, and the CRP level increased to 155 mg/L (range, <5.0 mg/L). Follow-up chest CT revealed submucosal fluid collection in the upper thoracic esophagus and large amounts of bilateral pleural effusion with passive atelectasis of both lower lobes (Fig. 3C). The antibiotic regimen was changed to piperacillin/tazobactam. Although the CRP level decreased to 21 mg/L (range, <5.0 mg/L) on HD 7, the patient’s fever persisted, and the antibiotics were changed to ceftriaxone on suspicion of drug-induced fever. The patient was allowed to sip water on HD 7. On HD 11, follow-up chest CT revealed decreased pleural fluid and esophageal submucosal fluid collection. On HD 22, a soft diet was initiated. Upon further improvement of the esophageal wall thickening, as observed on chest CT, the patient was discharged on HD 30 (Fig. 3D).
Although APE is very rare, it is life-threatening. The most important feature of APE is the spread of infection to the submucosa rather than the mucosa [1]. Early diagnosis of APE is very important as it enables treatment without surgery. Chest CT is the gold standard for diagnosing APE [2]. A characteristic feature of APE on CT is hypodense circumferential esophageal wall thickening with significant esophageal wall rim enhancement. Air bubbles within the thickened esophageal wall indicate infection by gas-producing pathogens, another characteristic of phlegmonous esophagitis [3,4].
The most common causative pathogens are Streptococcus spp., Staphylococcus spp., Escherichia coli, Haemophilus influenzae, Proteus spp., and Clostridiac [5]. The pathogen responsible for the phlegmonous infection in our cases was believed to be K. pneumoniae based on the culture results of the blood and pleural fluid. As empirical therapy, a combination of antibiotics capable of targeting anaerobes appeared reasonable.
APE is primarily treated with antibiotics, and intraluminal drainage may be helpful. Recently, Kim et al. [6] reported endoscopic mucosal dissection as a treatment for phlegmonous esophagitis, as it allows the pus of the submucosa to become intraluminal drainage. As presented in our first case, pus drainage by opening the esophageal mucosa in severe APE accompanied by neck infection helped alleviate the disease. In addition, sufficient nutritional support is required for recovery [2]. If central TPN is administered for an extended period owing to prolonged hospitalization, enteral feeding can facilitate adequate nutritional support. We performed enteral feeding through a nasojejunal tube on HD 9 because prolonged hospitalization was predicted for a patient with severe APE and neck infection who required more nutritional support. In the other two cases, APE was not very severe, and TPN was performed without enteral feeding. As tube insertion for feeding may damage the esophageal mucosa [2], enteral feeding was not performed during the early period of treatment in the case with severe inflammation and low APE severity.
Predisposing factors for APE include DM, alcoholism, old age, chronic gastritis, malnutrition, immunosuppression, and low socioeconomic status [4,7]. In our cases, all patients had type 2 DM. Two of the patients were younger than 40 years, and the incidence of APE at a young age is rare. Their predisposing factors included uncontrolled type 2 DM and chronic alcoholism. Despite the young age, poorly controlled DM may cause APE because numerous infections are associated with impaired carbohydrate tolerance [8].
Our observations demonstrate the recovery courses of patients with varying APE symptoms and severities who received medical treatment. Thus, it is important to reduce the need for risky surgical treatments in patients by using appropriate antibiotics, performing examinations such as chest CT and EGD at appropriate times, and performing appropriate medical procedures.
References
1. Kim HS, Hwang JH, Hong SS, Chang WH, Kim HJ, Chang YW, et al. Acute diffuse phlegmonous esophagogastritis: a case report. J Korean Med Sci. 2010; 25:1532–5.

2. Chang PC, Wang WL, Hwang TZ, Cheng YJ. Intramural dissection with mucosal rupture alleviating phlegmonous esophagitis. Eur J Cardiothorac Surg. 2012; 41:442–4.

3. Yun SM, Jeong YJ, Hwang M, Lee G, Lee JW, Kim GH, et al. Usefulness of contrast-enhanced CT in a patient with acute phlegmonous esophagitis: a case report and literature review. Medicina (Kaunas). 2022; 58:864.

4. Woo WG, Do YW, Lee GD, Lee SS. phlegmonous esophagitis treated with internal drainage and feeding jejunostomy. Korean J Thorac Cardiovasc Surg. 2017; 50:453–5.

5. Kim GY, Ward J, Henessey B, Peji J, Godell C, Desta H, et al. Phlegmonous gastritis: case report and review. Gastrointest Endosc. 2005; 61:168–74.

6. Kim JW, Ahn HY, Kim GH, Kim YD, Cho JS. Endoscopic intraluminal drainage: an alternative treatment for phlegmonous esophagitis. Korean J Thorac Cardiovasc Surg. 2019; 52:165–9.

7. Hsu CY, Liu JS, Chen DF, Shih CC. Acute diffuse phlegmonous esophagogastritis: report of a survived case. Hepatogastroenterology. 1996; 43:1347–52.
Fig. 1.
(A) Enhanced chest computed tomography (CT) shows circumferential wall thickening of the esophagus with multiple air bubbles. (B) Esophagogastroduodenoscopy (EGD) reveals esophageal mucosal edematous change and an upper opening. (C) On hospital day 5, chest CT reveals improvements in esophageal wall thickening, submucosal air, and fluid collection. (D) The previous opening appears as a pseudolumen on follow-up EGD. (E) Esophagogram reveals no passage disturbance or leakage.
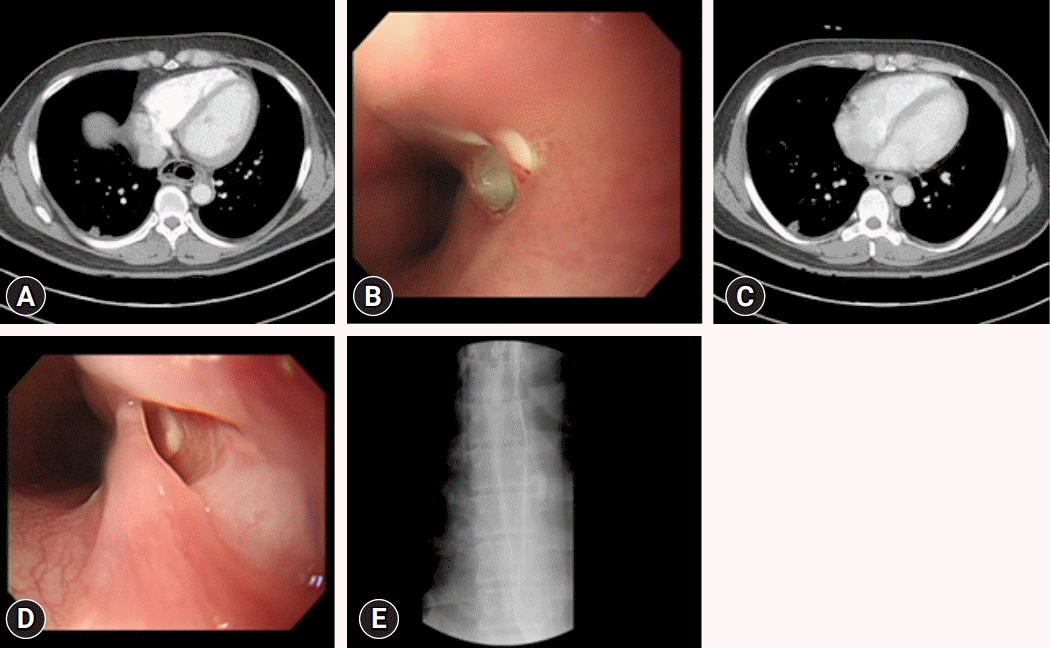
Fig. 2.
(A) Circumferential esophageal wall thickening and submucosal fluid collection with pleural effusion on initial chest computed tomography (CT) image. (B) Edematous esophageal mucosa on initial esophagogastroduodenoscopy (EGD) image. (C) Chest CT on hospital day (HD) 18. Improved esophageal wall edema and submucosal fluid collection. (D) EGD on HD 12. Esophageal wall edema is improved, and esophageal varices are seen on follow-up EGD.
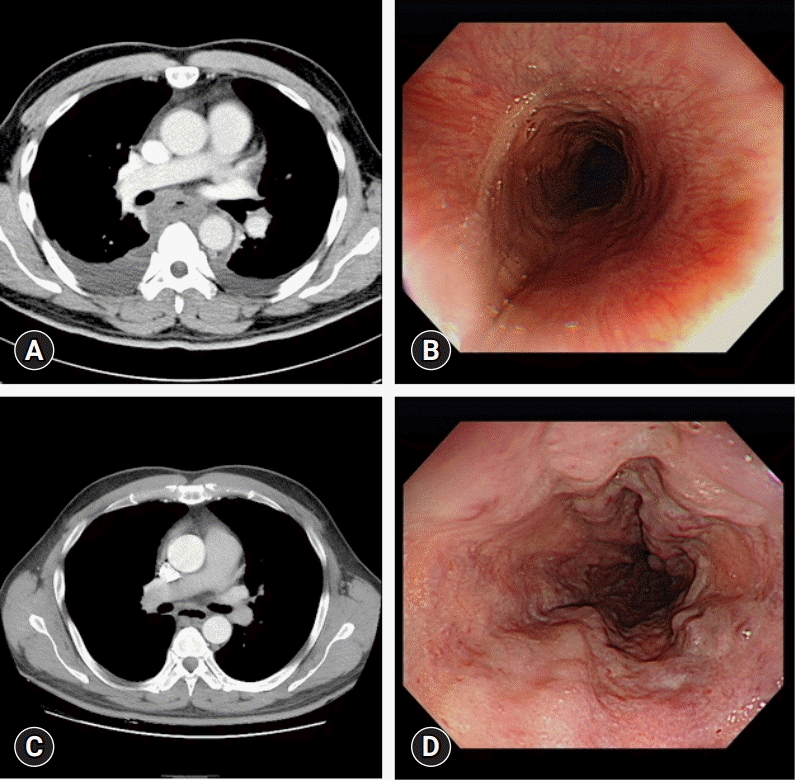
Fig. 3.
(A) Diffuse edematous esophageal wall thickening on the initial chest computed tomography (CT). (B) Edematous esophageal wall on initial esophagogastroduodenoscopy. (C) Chest CT on hospital day (HD) 5 reveals increased edema and submucosal fluid collection in the esophageal wall compared with the previous CT. (D) Chest CT on HD 29 reveals decreased esophageal wall thickening and submucosal fluid collection compared with the previous CT.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download