INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth leading cause of global cancer deaths [
1]. Despite significant advances in multimodal therapies such as surgical resection, transplantation, radiofrequency ablation, transarterial chemoembolization, sorafenib, and immune checkpoint inhibitor (ICI) throughout the decades, the long-term outcomes of HCC are still poor due to the high rates of locoregional and distant recurrence [
2]. Unfortunately, a significant proportion of patients are diagnosed with advanced clinical stages and underlying cirrhosis, where the rates of curative resection are very low. Recently, it has been noted that ICIs, including cytotoxic T-lymphocyte associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death-ligand 1 (PD-L1) inhibitors commonly used in the clinical setting have been developed and have shown significant oncological improvement in several cancers, such as melanoma, non-small cell lung cancer (NSCLC) and renal cell carcinoma, as noted in clinical trials. However, until now, the response rate of ICI for HCC still has been poor due to the incidence of intrinsic or acquired resistance [
3]. Therefore, focusing on identifying the molecular mechanisms involved in the low response rate and therapeutic resistance is needed in order to overcome the limitation.
Tumor-infiltrating immune cells (TIICs) have been used for the prediction of prognosis and treatment in cancer patients and they are crucial components of tumor microenvironments [
4]. Tumor-infiltrating lymphocytes (TILs), first described by Clark et al. [
5], are T cells, B cells, and natural killer cells that are the most widely studied populations of TIICs and their correlation with favorable outcomes in HCC has been reported previously [
6]. It is noted that the incidence of increased numbers of TILs, especially activated cytotoxic T lymphocytes (CTLs), is reported to correlate with favorable survival in some malignancies including HCC [
7]. However, the clinical significance of PD-L1+ TIICs in HCC is still considered controversial.
Epithelial-mesenchymal transition (EMT) is a key process in that epithelial cells lose the apical-basal polarity and cell-cell adhesion and transit to the invasive mesenchymal cells. It has been established to play a role in the diagnosis of embryogenesis, tissue fibrosis, cancer progression, and metastasis in patients. Here, EMT is induced by transcriptional factors (TF) such as transforming growth factor-β1 (TGF-β1), fibroblast growth factor, and so on, which can result in the loss of cell-cell adhesion occurring, alterations in EMT-associated markers including mesenchymal markers (N-cadherin, vimentin, Snail) and in an epithelial marker (E-cadherin). For this reason, the upregulation of mesenchymal markers in cancer cells acquires more metastatic potential [
8]. However, the potential molecular mechanisms of EMT-induced tumor immune suppression and evasion have not been well-known for HCC so far.
Recently, it has been noted that several groups reported a significant relationship between PD-L1 expression and EMT status [
9101112]. However, the clinical significance of PD-L1 expression on tumor cells (TCs) and immune cells (ICs), and EMT and the relationship between their expression and clinicopathological findings in patients with HCC have not been fully investigated to date in a Korean patient population. Therefore, in the current study, to explore the predictive role of expression of PD-L1 and EMT for HCC, we have especially focused on investigating the clinicopathological significance of PD-L1 on IC, TC, and EMT in formalin-fixed samples from 161 patients with HCC.
Go to :

METHODS
The Institutional Review Board of the Soonchunhyang University Cheonan Hospital approved the study (No. SCHCA 2020-03-024-004). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waved due to its retrospective nature.
Patients and samples
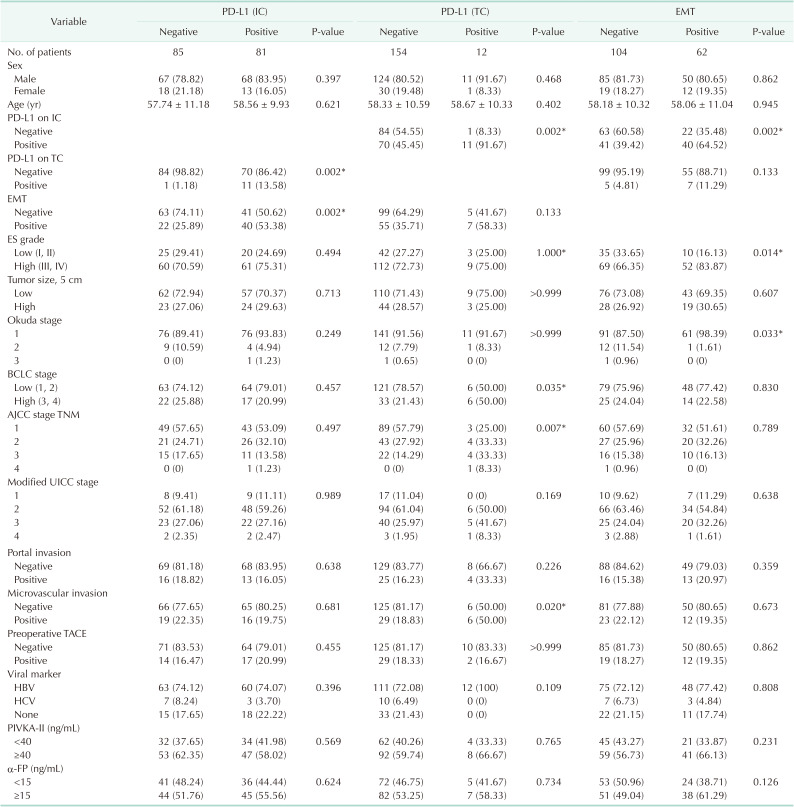
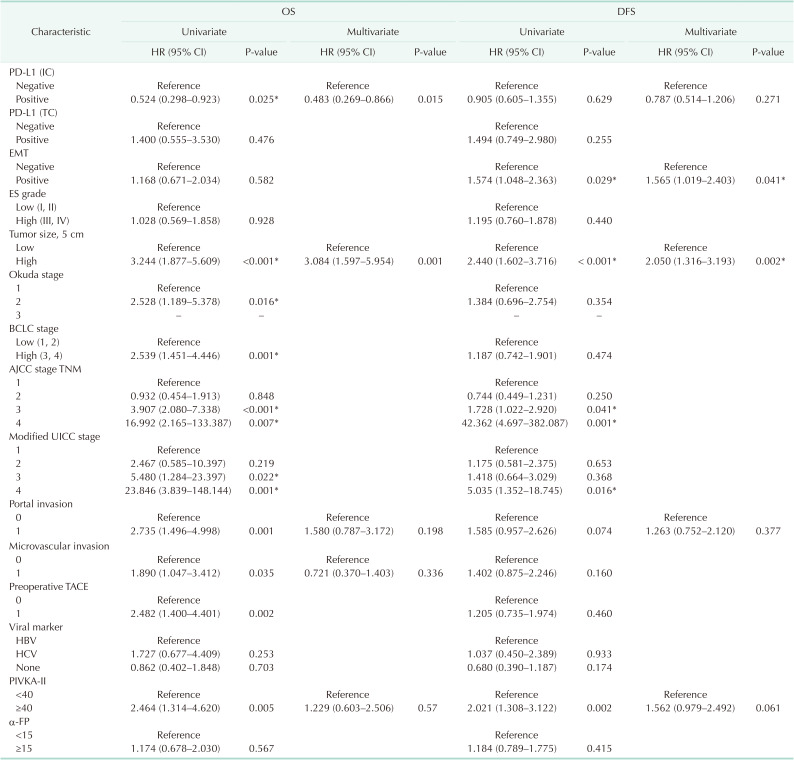
In this study, 166 patients who underwent microscopically complete curative resection such as wedge resection, segmentectomy, and hemihepatectomy between January 2009 and January 2015 at Soonchunhyang University Cheonan and Bucheon Hospitals with HCC, which were pathological confirmed and formalin-fixed, paraffin-embedded samples were selected. To this end, there were no patients who received preoperative chemotherapy or radiotherapy, and none had a distant metastasis. It is noted that no patient died within 30 days after surgery. Therefore, it is noted that the patients lost to follow up were not included. In the meantime, all of the clinicopathological data were collected by a retrospective review of the patient medical charts and pathological records. The clinicopathological parameters followed the rules for the study of primary HCC, 3rd ed, June 2007, Korea. Here, the tumor differentiation (grade) was assessed using the Edmondson and Steiner nuclear grading system (ES grade). Notably, the tumor stage was defined according to the 8th TNM classification of the American Joint Committee on Cancer (AJCC), Okuda staging, Barcelona Clinical Liver Cancer (BCLC) staging, and modified Union for International Cancer Control (UICC) staging.
Construction of tissue microarray
The H&E-stained slides made from formalin-fixed, paraffin-embedded tissue blocks were reviewed to select the most representative viable portion of the carcinoma. The corresponding areas of paraffin blocks were cored twice with a 2 mm-diameter cylinder and transferred to a recipient paraffin block using a tissue microarrayer (Unitma).
Immunohistochemistry and interpretation
Immunohistochemical staining was applied to each 4 µm-thick section from TMA blocks using the Ventana Benchmark XT automated staining system (Ventana Medical Systems) according to the manufacturer’s protocol. The following primary antibodies were used: anti-human E-cadherin mouse monoclonal antibody (clone 36, Ventana Medical Systems, ready to use), anti-vimentin monoclonal antibody (clone SRL33, diluted 1:200; Leica Biosystems) and PD-L1 antibody (rabbit monoclonal clone SP263, ref 790-4905; Ventana Medical Systems). Chorionic villi of human placenta were used as a positive control for PD-L1 antibody. In each core of TMA, 3 representative areas were selected and at least 100 TCs were observed at 400× magnification.
All immunohistochemical stained slides were blindly evaluated by an experienced pathologist (HA). PD-L1 expression was scored according to the proportion of PD-L1+ TCs (TC 0 for <1%, TC 1 for 1%–4%, TC 2 for 5%–49%, and TC 3 for ≥50%) and PD-L1+ ICs (IC 0 for <1%, IC 1 for 1%–4%, IC 2 for 5%–9%, and IC 3 for ≥10%). The expression of EMT markers of TCs was assessed using a semiquantitative scoring system. Immunoreactivity was defined as the number of TCs that had positive membranous staining patterns for E-cadherin and cytoplasmic staining for vimentin with minimal background staining. The intensity was scored as follows: 0, negative; 1, light brown staining (weak staining); 2, brown staining (moderate staining); and 3, dark brown staining (strong staining). The proportion was scored as follows: 0 for 0%–10%, 1 for 11%–25%, 2 for 26%–50%, 3 for 51%–75%, and 4 for 76%–100% (
Fig. 1). The final immunohistochemical score was calculated by multiplying the intensity and proportion scores. Final scores of 1 or more were considered positive for PD-L1 and vimentin. Tumors with a final score of E-cadherin ≤4 and >4 were regarded as tumors with low and high expression, respectively. EMT positive subgroup (EMT+) was defined by low expression of E-cadherin or positive expression of vimentin. EMT negative subgroup (EMT–) were included all other patients.
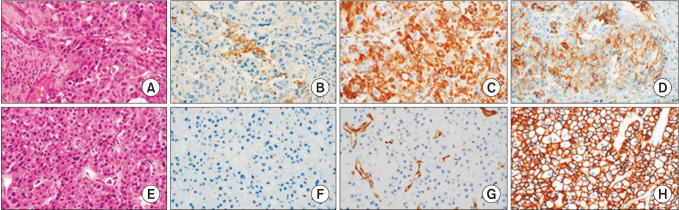 | Fig. 1Representative features of histologic and immunohistochemical analysis in high PD-L1+ immune cells/EMT+ case (A–D) and low PD-L1+ immune cells/EMT– case (E–H). (A and E: H&E stain, ×400; B and F: immunostaining of PD-L1, ×400; C and G: immunostaining of vimentin, ×400; D and H: immunostaining of E-cadherin, ×400). The EMT+ case shows strong cytoplasmic expression of vimentin and loss of membranous staining of E-cadherin and the EMT– case shows no expression of vimentin and strong membranous staining of E-cadherin. PD-L1, programmed cell death-ligand 1; EMT, epithelial-mesenchymal transition.
|
Statistical analysis
All of the data were analyzed by IBM SPSS Statistics ver. 26.0 (IBM Corp., Armonk, NY, USA) and with P-value of <0.05 as the threshold of statistical significance. The chi-square and Fisher exact tests were used to compare the levels of PD-L1, TILs, and EMT markers expression and the various clinicopathological characteristics between the groups. Survival curves for overall survival (OS) and disease-free survival (DFS) were calculated using the Kaplan-Meier method and were compared by the log-rank test. Multivariate analysis of prognostic relevance was evaluated by multivariate Cox regression analysis.
Go to :

DISCUSSION
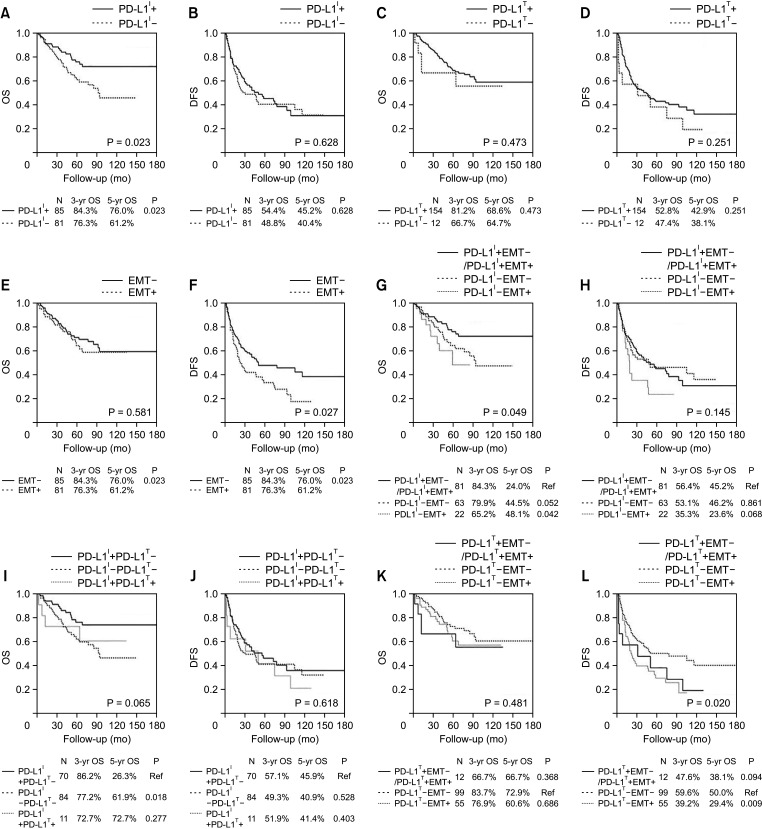
We investigated the expression of PD-L1 in TCs and ICs, as well as the expression of EMT markers, as independent prognostic markers in HCC patients. Analyzing the correlation between PD-L1 expression in TCs (PD-L1 TC) and clinicopathologic features suggests that tumor PD-L1 positivity might be associated with more aggressive tumor progression, including deeper tumor invasion and microvascular invasion. In other words, the expression of PD-L1 in ICs (PD-L1 IC) was significantly correlated and positively affected the survival of HCC. However, PD-L1 TC was not associated with patient survival. In this respect, some studies indicated that peritumoral PD-L1 expression was associated with significantly worse survival compared to the negative expression group [
13]. However, the results of some studies are characteristically seen with a reversed result. A recent meta-analysis which included 13 eligible studies containing 1,843 HCC patients, reported that PD-L1 seemed to function as a significant biomarker in the poor prognosis of HCC [
14]. In this regard, because the proportion of the positive group was very small (7%), our study might have shown that PD-L1 TC expression was not correlated with OS. On the other hand, we found out that the expression of PD-L1 IC which was one of the TIICs, in patients with HCC was correlated with better OS. Increased numbers of TILs, which were widely studied in other populations of TIICs, particularly activated CTLs, are correlated with a better survival rate in several cancer patients, including those with HCC [
15]. However, it is noted that several opposite results also were reported [
16]. Recently, 2 meta-analyses revealed that a high density of CD8+ TILs was associated with better OS and DFS, especially for Asian patients, and CD8+ TILs would serve as an effective marker for evaluating the prognosis of patients with HCC [
617].
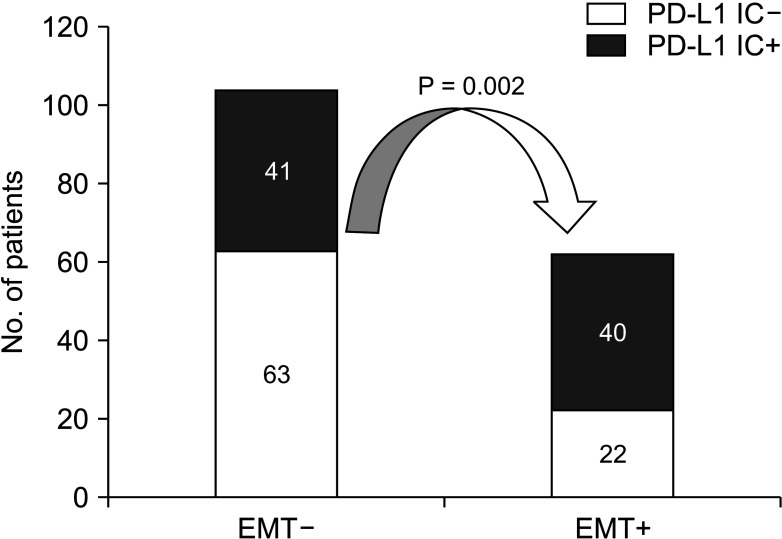
In our present study, the EMT status was significantly associated with recurrence in many cases. It is emphasized that we also observed that PD-L1 IC staining intensity was significantly lower in the EMT–, as compared to the EMT+ significantly represented in the data. It is also noted that these data indicate that EMT is one of the critical steps in the early stages of distant metastasis in HCC. In other words, once the cancer cells obtain mesenchymal phenotypes, they gain the ability to migrate into the stroma or migrate to distant sites and organs, and finally contribute to tumor metastasis and recurrence [
18]. Therefore, in these cases the epithelial phenotype is considered to be “stable,” whereas the mesenchymal phenotype is thought to be “metastable.” The mechanism of EMT modulation consists of complex networks involving epigenetic modification and transcriptional control, including EMT-inducing TFs (EMT-TFs) such as Snail, ZEB, and Twist and transcription regulators, such as represented with microRNAs [
19].
We evaluated here that PD-L1 expression of TC was associated with IC density. In addition, we found that the expression of PD-L1 IC was associated with a longer OS in patients who underwent surgery. We classified HCC tumors into 4 different types based on the presence or absence of PD-L1 IC and TC expression in the microenvironment. In these cases, type IV (PD-L1 TC– and IC+) showed a significantly better prognosis than type II (PD-L1 TC– and IC–). The proportions of patients with type I (PD-L1 TC+ and IC+) and type III (PD-L1 TC+ and IC–) as noted in a tumor microenvironment were very small (6.6% and 0.6%) and were lower than that found in other cancer such as advanced melanoma and esophageal squamous cell carcinoma (ESCC) [
20], and for patients of these 2 groups may be largely responding to ICI because of preexisting intra-tumoral T cells and promoted T-cell infiltration of tumors. For example, it was revealed that radiotherapy could induce immunogenic cell death to liberate neoantigens and promote T-cell infiltration of the tumor. In the meantime, we noted that the dominant type in HCC was type II and IV (50.6% and 42.2%) which was defined as PD-L1 TC– and IC–/+. Likewise, the proportions of these cells were higher than those found in other malignancies, such as melanoma and ESCC [
20]. Type II patients (PD-L1 TC– and IC–) which were believed to be fewer responding to single-agent ICI due to the lack of PD-L1 expression and preexisting T-cell infiltration [
21], could require more combination therapy, such as the combination of anti–CTLA-4 and anti–PD-1, to promote immune tolerance [
22]. Additionally, for type IV cancers (PD-L1 TC– and IC+), IC might be targeted by other non–PD-1/PD-L1 checkpoint receptors because of the immune ignorance state.
Moreover, we also investigated the clinical significance of EMT and PD-L1 expression of TC and IC in HCC. Here we found that the PD-L1 expression of IC is closely associated with EMT markers including E-cadherin and mesenchymal features. Patients who were PD-L1 IC+/EMT– showed a significantly better prognosis than those who were PD-L1 IC–/EMT+. Although PD-L1 expression of TC was not correlated with EMT, the recurrence rates were higher in the subgroup of PD-L1 TC–/EMT+ than in the subgroup of PD-L1 TC–/EMT–. This finding suggests that the incidence of PD-L1 positivity in TC and IC can be divided into subgroups based on EMT markers in HCC. The question then follows that these data indicate that EMT status may be a co-biomarker with PD-L1 to predict the prognosis of cancer and as a potential predictive biomarker to guide the selection of patients who are more likely to benefit from ICI. Interestingly, the incidence of a positive expression of PD-L1 IC does not guarantee a good response to PD-L1 inhibitors. This result could be due to the bidirectional regulation that might exist between PD-L1 expression and EMT status. Notably, there are several studies that have reported that PD-L1 has been considered as an important mediator of EMT in many cancers such as lung cancer, colorectal cancer, head and neck cancer, esophageal squamous cancer, breast cancer, extrahepatic cholangiocarcinoma, and so on [
3]. Some other reports indicated that PD-L1 signaling plays an important role in the maintenance of the EMT status in renal cell carcinoma, breast cancer, HCC, esophageal cancer, and glioblastoma [
23]. However, no studies have directly compared the EMT phenotype and PD-L1 expression of IC in HCC TCs from patient tissues. Thus, this study may be significant in that it may provide the first evidence that the EMT is associated with PD-L1 expression of TC and IC using HCC cases. Recent advances in The Cancer Genome Atlas (TCGA) data have also revealed that E-cadherin was strongly negatively correlated with PD-L1 protein expression. The PDL1+/EMT+ group exhibited poorer survival than the PD-L1+/EMT– patients in CGA cohort of lung adenocarcinoma and head and neck squamous cell carcinomas [
11]. Furthermore, elevated scoring of mesenchymal markers such as the Snail and vimentin was positively correlated with PD-L1 expression. It is shown that this may indicate that patients with mesenchymal phenotypes have an opportunity to have a more effective response to ICI.
It is important to note that the molecular mechanism for the relationship between PD-L1 and the EMT process remains unclear. Recent advances have revealed that the EMT process promotes upregulated expression of PD-L1 through several molecules such as mucin 1 (MUC1), nuclear factor κB (NF-κB), TGF-β, etc. [
2425]. Notably, with MUC1, a transmembrane glycoprotein, its upregulation in NSCLC is generally associated with a poor prognosis. This is because it activates the NF-κB p65/ZEB1 pathway to upregulate PD-L1 expression via NF-κB p65 occupancy on the promoter region of CD274 [
24]. TGF-β, a key inducer of EMT, also upregulates expression of PD-L1 dependent on PI3K/AKT and MEK/ERK pathways in breast cancer cells, and upregulation of PD-L1 is due to the EMT process but not TGF-β itself [
25]. In response to playing important roles of combination therapy for reinforcing the ICI, the key mediators including EMT-TFs, RAS/MAPK, PI3K/AKT/mTOR involved in the regulation of EMT-induced immune escape may represent potential novel therapeutic targets. A recently published Ib clinical trial applied cobimetinib combined with atezolizumab to investigate safety and clinical efficiency in solid tumors [
26]. The U.S. Food and Drug Administration approved nivolumab and pembrolizumab as second-line therapy for advanced HCC following the failure of sorafenib in September 2017 and November 2018, respectively. There are currently phase III trials of nivolumab and pembrolizumab (
NCT02576509, CheckMate-459, Keynote-240,
NCT02702401 and Keynote-394,
NCT03062358). However, many clinical trials are still phase I and II studies, and it is noted that ongoing phase III studies are also failing to produce satisfactory results. For this reason, further studies are urgently needed to explore and modify treatment strategies for anti–PD-1/PD-L1 therapy in combination with other targeting such as EMT. Recently, TGF-β inhibitors, M7824, and other novel EMT-targeted agents have been proven to exert synergistic therapeutic effects combined with PD-L1 inhibitors in previously published preclinical studies and clinical trials (
NCT02734160,
NCT02423343,
NCT03315871, and
NCT03493945). However, the immunotherapy and EMT targeted therapies are still in their infancy, most of these trials are phase I or II, and additional phase II or III clinical trials are needed to establish the clinical efficacy of these novel agents.
Unlike other cancers, in the case of liver cancer, adjunctive systemic chemotherapy following curative treatments such as surgery or radiofrequency ablation is not currently actively performed. The reason for this is that the therapeutic effect of systemic chemotherapy after liver cancer surgery has not yet been definitively proven to decrease recurrence rates [
27]. However, if more effective treatments for liver cancer are developed in the future, they are expected to be introduced not only for metastatic liver cancer but also for adjuvant therapy after surgery, leading to an increase in the OS rate of liver cancer patients. In this context, research is needed to determine the effectiveness of drugs that utilize the mechanism of immune checkpoint inhibition, in addition to existing drugs that block angiogenesis like sorafenib and ramucirumab, in liver cancer.
In this study, it was shown that liver cancer patients with PD-L1 TC expression have a poor prognosis in terms of DFS, and the expression of PD-L1 in ICs is associated with a favorable prognosis in terms of OS. This result provides a basis for introducing anti–PD-1/PD-L1 treatments in the management of liver cancer.
One limitation of this study is the lack of novelty. Although research on ICIs in HCC is rare, clinical trials for the anti–PD-1 inhibitors nivolumab and pembrolizumab were announced in 2010 and 2014, respectively, and they have already been approved for the treatment of melanoma and lung cancer. Secondly, we noted that the retrospective design and relatively small number of samples with a heterogeneous clinical status used in the current study could potentially bias the results. Therefore, the predictive role of PD-L1 expression of TC and IC is still considered controversial at this time. For this reason, future studies should involve increased numbers of cases in review at multiple institutions.
In conclusion, this study demonstrates that PD-L1 expression of ICs is closely associated with EMT marker expression in HCC. Patients who were PD-L1 IC+/EMT+/– showed a significantly better prognosis than those who were PD-L1 IC–/EMT+. Therefore, Clinical investigations using anti–PD-1/PD-L1 inhibitors in patients with EMT-associated PD-L1 upregulation are warranted. In the future, investigating the molecular mechanisms involved in primary or acquired resistance to anti–PD-1/PD-L1 therapy and the role of EMT in this process should be a key issue.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download