Abstract
In recent years, the rise of minimally invasive surgery has driven the development of surgical devices. Indocyanine green (ICG) fluorescence imaging is receiving increased attention in colorectal surgery for improved intraoperative visualization and decision-making. ICG, approved by the U.S. Food and Drug Administration in 1959, rapidly binds to plasma proteins and is primarily intravascular. ICG absorption of near-infrared light (750–800 nm) and emission as fluorescence (830 nm) when bound to tissue proteins enhances deep tissue visualization. Applications include assessing anastomotic perfusion, identifying sentinel lymph nodes, and detecting colorectal cancer metastasis. However, standardized protocols and research on clinical outcomes remain limited. This study explores ICG’s role, advantages, disadvantages, and potential clinical impact in colorectal surgery.
In recent years, minimally invasive surgery has been steadily on the rise, leading to the development of various surgical devices to accommodate this trend [123456]. There has been a growing interest in the application of indocyanine green (ICG)-fluorescence imaging (FI) in colorectal surgery [789]. This innovative technology has shown great promise in enhancing intraoperative visualization and decision-making, ultimately leading to improved outcomes for patients undergoing colorectal surgery.
ICG, which is a water-soluble tricarbocyanine compound, was originally approved by the U.S. Food and Drug Administration in 1959, it was primarily used for hepatic function diagnostic tests. In the early 1970s, its applications extended to retinal angiography. Upon intravenous injection, ICG quickly and extensively binds to plasma proteins, predominantly remaining within the intravascular space. Its half-life ranges from 3 to 5 minutes, followed by hepatic clearance and excretion into the bile [2], and the manufacturers advise not exceeding a daily dose of 2 mg/kg [3]. ICG possesses the unique ability to absorb near-infrared (NIR) light at wavelengths between 750 and 800 nm and emit it as fluorescence at 830 nm when bound to proteins in tissue [6]. This property proves particularly valuable for investigating deep tissues and structures.
ICG guidance into colorectal surgery presents several useful applications [6789]. Firstly, it aids in assessing vascular perfusion in colorectal anastomoses, enabling surgeons to make informed decisions about the viability of the anastomotic site. This is crucial in preventing anastomotic leakage. Additionally, ICG has been invaluable in the identification of sentinel lymph nodes (SLNs) during colorectal cancer (CRC) surgery, allowing for accurate staging and tailored treatment approaches. Furthermore, ICG-FI provides detection of metastasis in CRC.
However, the protocols for emerging techniques are less standardized, and there is a paucity of research on their impact on clinical outcomes. This descriptive review aimed to explore the application of ICG in colorectal surgery, its respective advantages and disadvantages, and its potential impact on clinical outcomes.
ICG-FI allows for visualization of tissue perfusion under NIR light. The injection of ICG provides real-time identification of bowel perfusion intraoperatively and this property would be useful in preventing anastomotic leakage (AL). AL is one of the major complications that might influence oncological and functional outcomes [1011]. Among factors associated with AL [12131415], poor bowel perfusion is the most important factor associated with AL in colorectal surgery [16]. Various method has been attempted to reduce AL or sequel of AL [17181920].
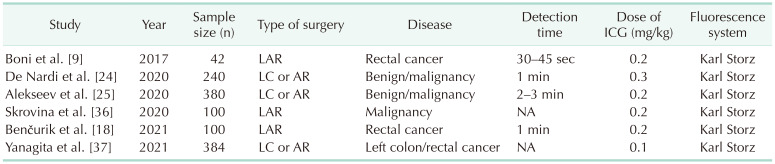
With ICG-FI, delineating the demarcation between vascular and avascular segments and establishing the appropriate transection margin may serve as a viable strategy to create a well-perfused anastomosis, thereby reducing the incidence of AL (Fig. 1). The area where surgeons use ICG most frequently in colorectal surgery is bowel perfusion assessment [17181920] (Table 1).
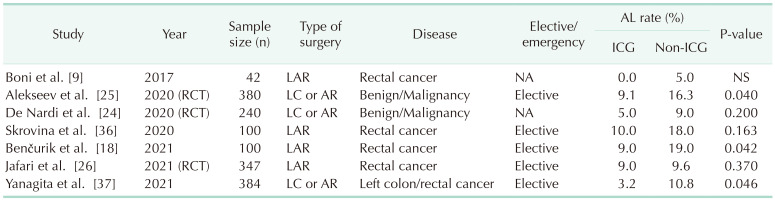
Some cohort studies and meta-analyses have reported an effect of ICG-FI for a decrease in AL [18,19,20,21,22,23]. One of these meta-analyses reported that the surgical plan of 9.6% of patients was changed based on ICG-FI, and using ICG-FI was associated with significantly lower odds of AL (odds ratio [OR], 0.452; 95% confidence interval [CI], 0.366–0.558) [22]. Nevertheless, the impact of ICG-FI on decreasing the rate of AL has yielded inconsistent findings. Two randomized controlled trials (RCTs) were conducted concurrently, with one reporting a decrease in AL, in line with prior research, while the other observed no reduction in AL [2425]. In 2021, another RCT reported that there was no difference in AL rate between patients who underwent perfusion assessment and those who underwent the standard surgical technique [26]. Several factors may contribute to these conflicting results observed across various studies. The risk factors associated with AL are multifactorial and encompass variables such as location, gender, receipt of preoperative chemoradiotherapy, preoperative obstruction, and technical factors [2728]. These factors may vary across studies, making it challenging to isolate the specific benefits of a single factor, such as perfusion assessment via ICG-FI.
Another contributing factor to the disparate findings is the variability in protocols employed across different studies (Table 2). Thus far, there has been no standardization in the use of ICG and, as a result, it often fails to provide quantitative data for precise perfusion assessment. Consequently, the determination of the transection point during surgery may exhibit subjectivity. Furthermore, experienced surgeons may derive fewer advantages from ICG-FI for assessing bowel perfusion compared to their less-experienced counterparts. Currently, 3 RCTs are ongoing ([29], ICG-COLORAL [NCT03602677], and InTACT trial [ISCRN 13334746]), and it will be necessary to examine the results of these studies in the future.
ICG-FI, especially when performing intracorporeal anastomosis (IcA), aids in perfusion assessment. While IcA (ICG angiography) has demonstrated an increase in utility, it has its limitations. One such limitation is the inability to directly palpate the anastomosed intestine or vessel and directly observe the actual color tone. Hence, in the context of IcA, assessing bowel perfusion with ICG-FI is likely to offer more valuable insights. In a retrospective study, ICG-FI detected inadequate anastomotic perfusion in 5.8% (4 of 69 patients) of cases within the intracorporeal group, whereas none were identified in the extracorporeal group (P = 0.046) [30]. The use of ICG-guided surgery can enhance the assessment of anastomotic perfusion and complement the capabilities of IcA. ICG-FI can also be applied in pouch surgery, which is usually done in total proctocolectomy for ulcerative colitis or familial adenomatous polyposis. When making a pouch, if the mesentery is short, it can pose challenges in achieving a tension-free anastomosis. To address this issue, mesentery lengthening can be achieved by ligating the ileocolic vessel or intermediate branches of the superior mesenteric vessel. However, it is important to note that during mesenteric lengthening techniques, there is a risk of perfusion deterioration, which can increase the rate of pouch-related complications, potentially reaching up to 20% [31]. Hence, using ICG-FI for perfusion assessment during mesentery lengthening may mitigate the risk of pouch failure. In a retrospective study involving 16 patients with ileal pouch-anal anastomosis, perfusion was assessed using ICG in both intraluminal and extraluminal pouch. Notably, there were no cases of pouch ischemia; however, 2 cases of AL were reported [32].
As mentioned earlier, the absence of standardized protocols for the application and interpretation of ICG is the foremost issue affecting the practical implementation of this technique. One limitation of ICG-guided surgery for perfusion assessment is the subjective judgment of the surgeon. Surgeons may interpret ICG-FI differently when evaluating bowel perfusion, potentially resulting in variations in their interpretations [33]. To overcome the drawback of subjectivity, several quantitative assessments were introduced, measuring fluorescence intensity [3435].
Additionally, the dosage and timing of evaluations constitute another crucial concern. In multiple studies, the typical dosage commonly used was in the range of 0.2–0.3 mg/kg [18242536]. The identification of bowel perfusion can typically be observed within a timeframe ranging from 30 seconds to 3 minutes [918242537]. ICG was primarily employed to determine the bowel transection line. ICG is injected intravenously after the division of the mesentery at the level of the planned transection. After ICG injection, vascular perfusion was then observed by an NIR camera system within seconds to minutes. If uniform blue light emission was detected and a clear demarcation line appeared, bowel transection was performed guided by ICG-FI. If vascular perfusion was judged to be poor, the transection line of the bowel would be changed to another site with good vascular perfusion under the NIR camera system, with an anastomosis fashioned at the new transection plane. Some studies have reported introducing an NIR scope through a transanal approach for intraluminal assessment after colorectal anastomosis, demonstrating that it is safe and feasible to evaluate mucosal perfusion of the anastomosis [3839].
When ICG is administered via injection into the submucosal or subserosal layers surrounding the tumor, it enables the visualization of lymphatic structures through NIR imaging [40]. ICG injected near the tumor is taken up by lymphatics and binds to protein to travel to the lymph nodes where it is deposited in macrophages [41]. Theoretically, ICG-FI provides a lymphatic map that accentuates the lymphatic drainage pathways originating from the primary tumor within the mesentery (Fig. 2).
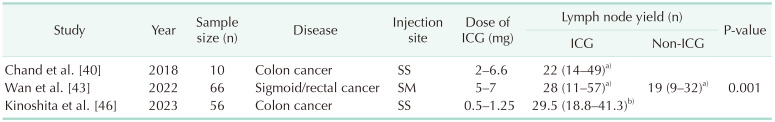
This lymphatic mapping can assist in identifying the optimal mesocolic resection margin, leading to the retrieval of optimized lymph nodes and potentially improving overall survival (Table 3).
Two prospective trials have demonstrated clinical outcomes of ICG lymphatic mapping in CRC surgery. The GREENLIGHT trial was designed to investigate the clinical relevance of a D3 ICG-guided lymph node dissection in CRC. An interim analysis of the trial on the first 70 cases was reported in 2022 [42]. ICG lymphatic mapping identified the aberrant lymph nodes and changed in the extension of the D3 lymphadenectomy in 50% of patients that would have been performed based on anatomical landmarks under white light vision. In another prospective randomized trial involving D3 lymph node dissection in sigmoid and rectal cancer, the ICG-guided surgery group, as compared to the conventional group using white light laparoscopy, harvested an additional 2 lymph nodes. Nevertheless, there was no significant difference in metastatic lymph node yields [43]. These studies demonstrated the feasibility of using ICG-guided surgery for D3 lymph node dissection in CRC surgery [4243].
Preoperative or intraoperative ICG injections are performed for lymphatic mapping and sentinel node identification [41424445]. Preoperative ICG injection entails the endoscopic submucosal injection of ICG for 1 to 2 days prior to surgery. ICG is injected into the submucosal layer in close proximity to the tumor, using an injection needle under colonoscopic guidance. Intraoperatively, ICG is injected into the subserosa near the tumor, and subsequent to the subserosal injection during surgery, lymphatic mapping can be discerned within a few minutes. The time required for ICG to reach and be identified in the lymph nodes can vary, as reported in studies, with one study suggesting that this process is frequently observed within a timeframe of 30–60 minutes [46]. ICG dosages have varied widely, ranging from 1 to 20 mg. However, in recent studies, it has been reported that approximately 0.2–0.5 mL of ICG solution (2.5 mg/mL) is commonly employed [41444546] (Fig. 3).
The SLN is defined as the initial node in the nearby lymphatic system that receives drainage from the primary tumor. Techniques such as radioisotope or dye injection are used for its identification. In the context of breast cancer, a SLN biopsy (SLNB) is commonly performed to assess the status of axillary lymph nodes in cases where there is no clinical evidence of lymph node involvement. Recently, the concept of SLNB has been expanded to include gastrointestinal cancers [484950]. This approach offers a significant advantage in CRC by enabling the detection of metastases and micro-metastases through a focused examination of a limited number of lymph nodes, theoretically. This improved accuracy aids in the staging of CRC. Detection rate and sensitivity, however, were not enough to apply this method in clinical practice.
Some recent meta-analyses have reported on the detection rates and sensitivities of SLN mapping. In the meta-analysis conducted by Ankersmit et al. [48], which focused on laparoscopic colon cancer surgery, a detection rate of 89.7% and sensitivity of 44.0% were reported. Another meta-analysis conducted by Villegas-Tovar et al. [49] reported a detection rate of 91.0% and a sensitivity of 64.3%. Notably, colon cancer exhibited significantly higher sensitivity in comparison to colorectal and rectal cancer with an estimated OR of 0.655 (95% CI, 0.548–0.762; P < 0.001). Additionally, laparoscopic surgery demonstrated higher sensitivity than open surgery, with an estimated OR of 0.677 (95% CI, 0.543–0.812; P < 0.001). Burghgraef et al. [50] compared early and advanced T-stage tumors. They reported detection rates and sensitivities of 91.0% and 80.0% for T1–T2 tumors, and 90.0% and 30.0% for T3–T4 tumors, respectively. These findings underscore the effectiveness of SLN mapping, particularly in the laparoscopic approach and early T-stage colon cancer. While researchers have made efforts to assess the efficacy of SLNB in colorectal surgery, it’s crucial to acknowledge that sensitivity values reported in these studies display significant variability. Consequently, there is an ongoing need for higher-quality research to provide a more comprehensive evaluation of the utility of ICG SLN mapping.
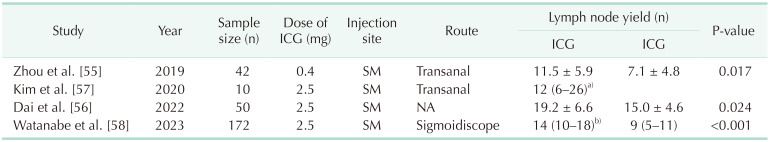
While lateral pelvic lymph node (LPLN) metastasis was traditionally viewed as systemic metastasis in rectal cancer, there has been a growing recognition that neoadjuvant chemoradiotherapy alone may not be sufficient in preventing lateral pelvic recurrence. Consequently, there has been a significant surge in interest regarding the role of LPLNs in the treatment of rectal cancer [51525354]. The lateral pelvic wall has a narrow and deep configuration, densely filled with blood vessels and nerve plexuses. As a result, identifying enlarged LPLNs and dissecting the lymph nodes without injuring other structures can be challenging, and identification of involved lymph nodes can also pose difficulties. However, with ICG-guided surgery, intraoperative blood loss can be reduced, and a greater number of LPLNs can be harvested [5556] (Table 4). Furthermore, by applying 3-dimensional (3D) reconstruction and ICG image-guidance technique for LPLN dissection (LPLND), accurate localization of suspicious LPLNs would be achieved. The suspicious LPLNs among ICG-bearing lymph nodes can be definitely identified intraoperatively by comparing them with the preoperatively reconstructed 3D images [57]. Thereby, ICG-guided surgery offers advantages in terms of surgery accuracy, completeness, and safety in LPLND. However, it’s important to note that the presence of ICG in a lymph node does not guarantee that it is a metastatic lymph node. Hence, the translation of obtaining a greater number of LPLNs with ICG guidance into a direct oncologic benefit necessitates further investigation.
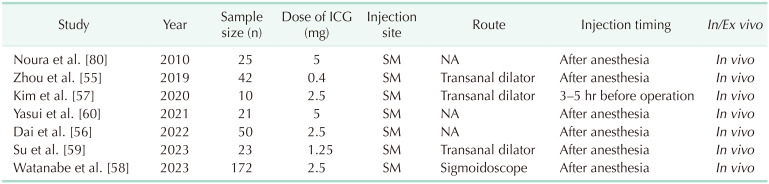
There is little study on oncologic outcomes according to employing the ICG technique. Watanabe et al. [58] reported the long-term oncologic outcomes of ICG-guided LPLND, revealing a 3-year cumulative lateral local recurrence rate of 0% in the ICG group compared to 9.3% in the non-ICG group. This study demonstrated that ICG-guided laparoscopic LPLND results in a lower rate of lateral local recurrence. The indication for LPLND can be determined through SLNB of LPLNs. Several studies adopted a procedure in which ICG is injected after the induction of general anesthesia, with the patient placed in the lithotomy position (Table 5). Using either an anal dilator or sigmoidoscope, a total of 1.0–1.5 mL of ICG (25 mg/10 mL) is locally injected into the submucosal layer, divided into 3–4 injections around the tumor [565859].
In recent studies, patients with rectal cancer and no evidence of lymph node enlargement underwent SLNB followed by LPLND. Among patients without SLN metastasis, all dissected lateral non-SLNs were found to be negative, indicating the absence of false-negative cases [5960]. This suggests the possibility of proposing a strategy for determining the need for LPLND through SLNB. However, the sample size in these studies was lower than 30 cases, so further large cohort research is needed.
Intraoperative detection of peritoneal metastasis (PM) currently relies on a surgeon’s manual palpation and visual assessment while staging laparoscopy and laparotomy. However, some small nodules can be missed during visual inspection. Disease progression can occur shortly after surgery from these peritoneal implants.
The use of new diagnostic imaging techniques such as ICG-FI could clearly increase the intraoperative detection of PMs allowing accurate, complete surgical resection and possibly improving the prognosis of patients. In 2013, Satou et al. [61] first reported that peritoneal metastases from hepatocellular carcinoma accumulate ICG after intravenous injection, emit fluorescence, and can be detected by NIR camera. Subsequently, Barabino et al. [62] and Liberale et al. [63] reported that peritoneal carcinomatosis from CRC can be visualized intraoperatively using ICG-FI.
Although ICG is not a cancer-specific binding molecule, it allows for the identification of cancer by accumulating in it and emitting fluorescence. The mechanism most frequently introduced in the literature to explain this phenomenon is the enhanced permeability and retention (EPR) effect in solid tumors [6465]. The tumors often exhibit distinct pathophysiological characteristics that are not present in normal tissues or organs. These include features like extensive angiogenesis leading to hypervascularization, flawed vascular structure, compromised lymphatic drainage systems, and a substantial elevation in the production of various permeability mediators. The phenomenon recognized as the EPR effect has been universally observed in solid tumors for lipid and macromolecular agents. ICG primarily binds to serum albumin and other serum globulins after intravenous injection, behaving like a macromolecule as it circulates. The EPR effect leads to the leakage of ICG-bound molecules into the extravascular space. Extravascular ICG accumulation allows for the detection of tumors by emitting fluorescence that contrasts with the surrounding normal tissue [66].
Some reports have indicated that the utilization of ICG-FI leads to more precise detection of PM. This allows for more patients to be optimally staged, potentially leading to suitable treatment and improved overall survival rates.
A pilot study examined 63 peritoneal resected nodules with the ICG-FI method in 14 patients, revealing that 84% of these were malignant. This study demonstrated that nonmucinous PM of CRC can be visualized intraoperatively using ICG-FI [63]. NIR probe is capable of detecting fluorescent lesions of approximately 1–2 mm in size [62]. In cytoreductive surgery, cytoreduction aims to achieve a residual tumor size no larger than 2.5 mm. The completeness of cytoreduction is one of the most important prognostic factors for survival in peritoneal carcinomatosis [67]. A prospective study reported that sensitivity increased from 76.9% with conventional diagnostic procedures to 96.9% with ICG-FI, indicating that intraoperative ICG-FI can enhance the completeness of cytoreduction [68]. To detect small lesions objectively, a quantitative assessment of ICG-FI was evaluated and, in a recent study, it has been reported that an ICG uptake value of 181 units or higher is indicative of a high likelihood of malignancy [69]. A recent systematic review highlighted that ICG-FI for peritoneal carcinomatosis is a promising intraoperative tool for the detection of peritoneal seedings. However, it’s important to acknowledge that significant variability in the sensitivity of ICG-FI for the detection of PM is observed, with reported values spanning from 72.4% to 96.9% [70]. Furthermore, some studies have reported the detection of PMs as small as 1–2 mm [6263]; however, there remains controversy regarding whether ICG-based detection is superior to visual inspection during laparoscopy or laparotomy for detecting very small lesions.
There are very limited data about ICG dosage and imaging timing for detecting PMs from CRC. In most studies, 0.25 mg/kg of ICG was injected intravenously, and the injection timing varied depending on the study [62636869]. Barabino et al. [62] administered ICG 24 hours before surgery and reported a sensitivity of 72.4%. González-Abós et al. [69] administered ICG 12 hours before surgery and performed intraoperative quantitative assessment. They considered it malignant when it measured above 181 units, resulting in a sensitivity of 89.0%. Liberale et al. [63] and Lieto et al. [68] administered ICG intraoperatively after complete exposure of the abdominal cavity and reported sensitivities of 87.5% and 96.9%, respectively. Further research is needed on the optimal injection timing and dosage of ICG for detecting PMs.
Resectability of CRC liver metastasis (CRLM) plays a crucial role in the outcome [7172]. However, even when CRLM is considered resectable before surgery, surgeons often face challenges in accurately identifying these metastases during the surgical procedure. It is well known that intraoperative ultrasound (IOUS) offers identification of CRLM undetectable by the naked eye [73]. ICG-FI has also been demonstrated as an effective tool for detecting hepatic metastasis [66]. To enhance the detection of metastatic liver lesions in CRC patients, a combined approach utilizing IOUS and ICG-FI was introduced and examined [74]. This study revealed that the combination of ICG-FI and IOUS outperforms IOUS alone, particularly in the detection of lesions smaller than 3 mm [74]. ICG accumulates within cancerous tissues following intravenous administration before surgery, making ICG-FI applicable for the detection of CRLM. The presence of ICG in metastatic liver tumors can be attributed to disruptions in biliary excretion. These disruptions may arise from structural obstructions within the biliary system or a functional decline in biliary transport. As a result, ICG accumulates within cancerous tissues. A recent clinical trial demonstrated improved oncologic outcomes with a lower 1-year recurrence rate in the ICG-FI group compared to the non–ICG-FI group [75]. However, a significant limitation of this approach is its ability to detect only lesions on the liver surface. Further research is warranted to address and overcome this limitation.
Minimally invasive surgery presents challenges in the identification of neoplasms due to the absence of tactile sensation. It is important to identify the tumor’s location to ensure adequate margins for resection and lymphadenectomy. To achieve tumor localization, preoperative endoscopic tattooing has been employed, particularly in cases of early colon cancer and radical surgery following incomplete endoscopic resection. Several substances have served as markers, including methylene blue, indigo carmine, and ICG. However, only India ink and ICG have demonstrated visibility for up to 48 hours after injection [76]. Miyoshi et al. [77] reported that they injected 12.5 mg of ICG near the tumor using an endoscope before surgery, and they were able to identify the marking on the white light image during laparoscopic surgery. During the same period, Watanabe et al. [78] demonstrated that FI allows for detection with lower concentrations of ICG, which is 1.25 mg. In a recent meta-analysis, it was noted that several studies employed the ICG injection method in the following manner: ICG (25 mg) was mixed with sterile water or normal saline, yielding a final solution with concentrations ranging from 2.5 to 12.5 mg/mL. The injection volume varied from 0.1 to 1.5 mL of the solution, administered at 1–4 sites near the tumor [79]. Considering ICG can be applied multifunctionally, such as lymphatic mapping and angiography, during a single surgery, Ahn et al. [41] suggested that 0.5–1 mg of ICG for preoperative endoscopic tattooing may be optimal. Using ICG-FI is a reliable approach for marking the tumor site, provided that ICG is injected into the submucosal layer around the tumor before laparoscopic or robotic colorectal surgery.
ICG-FI offers valuable visualization of vessels, lymphatics, and tumors in colorectal surgery. Its application has demonstrated promise in achieving favorable clinical outcomes, including a reduction in AL and improved lymphadenectomy. These findings emphasize the potential advantages of incorporating ICG-FI into colorectal surgical practice, providing enhanced intraoperative guidance and potentially leading to improved patient outcomes. However, for practical utilization, standardization of protocols is essential, and the development of objective evaluation methods is necessary. Additionally, in the context of tumor surgery, it is important to recognize that the presence of tumors visualized with ICG does not necessarily indicate metastasis. Therefore, careful consideration is required to determine how to utilize ICG not merely for the purpose of achieving more extensive resections but rather to enhance oncological outcomes.
References
1. Shah MF, Naeem A, Haq IU, Riaz S, Shakeel O, Panteleimonitis S, et al. Laparoscopy offers bet ter clinical outcomes and long-term survival in patients with right colon cancer: experience from national cancer center. Ann Coloproctol. 2022; 38:223–229. PMID: 34167186.

2. Son GM, Kwon MS, Kim Y, Kim J, Kim SH, Lee JW. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc. 2019; 33:1640–1649. PMID: 30203201.

3. Cahill RA, Ris F, Mortensen NJ. Near-infrared laparoscopy for real-time intra-operative arterial and lymphatic perfusion imaging. Colorectal Dis. 2011; 13 Suppl 7:12–17. PMID: 22098511.

4. Piozzi GN, Kim SH. Robotic intersphincteric resection for low rectal cancer: technical controversies and a systematic review on the perioperative, oncological, and functional outcomes. Ann Coloproctol. 2021; 37:351–367. PMID: 34784706.

5. Ryu HS, Kim J. Current status and role of robotic approach in patients with low-lying rectal cancer. Ann Surg Treat Res. 2022; 103:1–11. PMID: 35919115.

6. Cahill RA, Anderson M, Wang LM, Lindsey I, Cunningham C, Mortensen NJ. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc. 2012; 26:197–204. PMID: 21853392.

7. Son GM, Ahn HM, Lee IY, Ha GW. Multifunctional indocyanine green applications for fluorescence-guided laparoscopic colorectal surgery. Ann Coloproctol. 2021; 37:133–140. PMID: 34102813.

8. Ahn HM, Son GM, Lee IY, Park SH, Kim NS, Baek KR. Optimization of indocyanine green angiography for colon perfusion during laparoscopic colorectal surgery. Colorectal Dis. 2021; 23:1848–1859. PMID: 33894016.

9. Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc. 2017; 31:1836–1840. PMID: 27553790.

10. Kim S, Kang SI, Kim SH, Kim JH. The effect of anastomotic leakage on the incidence and severity of low anterior resection syndrome in patients undergoing proctectomy: a propensity score matching analysis. Ann Coloproctol. 2021; 37:281–290. PMID: 34098631.

11. Koedam TW, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, et al. Oncological outcomes after anastomotic leakage after surgery for colon or rectal cancer: increased risk of local recurrence. Ann Surg. 2022; 275:e420–e427. PMID: 32224742.
12. Chaouch MA, Kellil T, Jeddi C, Saidani A, Chebbi F, Zouari K. How to prevent anastomotic leak in colorectal surgery?: a systematic review. Ann Coloproctol. 2020; 36:213–222. PMID: 32919437.

13. Mizuuchi Y, Tanabe Y, Sada M, Tamura K, Nagayoshi K, Nagai S, et al. Cross-sectional area of psoas muscle as a predictive marker of anastomotic failure in male rectal cancer patients: Japanese single institutional retrospective observational study. Ann Coloproctol. 2022; 38:353–361. PMID: 35410111.

14. Oh BY, Park YA, Huh JW, Cho YB, Yun SH, Kim HC, et al. Neoadjuvant chemoradiotherapy determines the prognostic impact of anastomotic leakage in advanced rectal cancer. Ann Surg Treat Res. 2022; 103:235–243. PMID: 36304190.

15. Degiuli M, Elmore U, De Luca R, De Nardi P, Tomatis M, Biondi A, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): a nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Colorectal Dis. 2022; 24:264–276. PMID: 34816571.

16. Varela C, Nassr M, Razak A, Kim NK. Double-layered hand-sewn anastomosis: a valuable resource for the colorectal surgeon. Ann Coloproctol. 2022; 38:271–275. PMID: 35295072.

17. Crafa F, Striano A, Esposito F, Rossetti AR, Baiamonte M, Gianfreda V, et al. The “reverse air leak test”: a new technique for the assessment of low colorectal anastomosis. Ann Coloproctol. 2022; 38:20–27. PMID: 33332954.

18. Benčurik V, Škrovina M, Martínek L, Bartoš J, Macháčková M, Dosoudil M, et al. Intraoperative fluorescence angiography and risk factors of anastomotic leakage in mini-invasive low rectal resections. Surg Endosc. 2021; 35:5015–5023. PMID: 32970211.

19. Alekseev M, Rybakov E, Khomyakov E, Zarodnyuk I, Shelygin Y. Intraoperative fluorescence angiography as an independent factor of anastomotic leakage and a nomogram for predicting leak for colorectal anastomoses. Ann Coloproctol. 2022; 38:380–386. PMID: 34289650.

20. Dinallo AM, Kolarsick P, Boyan WP, Protyniak B, James A, Dressner RM, et al. Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks?: a retrospective cohort analysis. Am J Surg. 2019; 218:136–139. PMID: 30360896.

21. Renna MS, Grzeda MT, Bailey J, Hainsworth A, Ourselin S, Ebner M, et al. Intraoperative bowel perfusion assessment methods and their effects on anastomotic leak rates: meta-analysis. Br J Surg. 2023; 110:1131–1142. PMID: 37253021.

22. Emile SH, Khan SM, Wexner SD. Impact of change in the surgical plan based on indocyanine green fluorescence angiography on the rates of colorectal anastomotic leak: a systematic review and meta-analysis. Surg Endosc. 2022; 36:2245–2257. PMID: 35024926.

23. Trastulli S, Munzi G, Desiderio J, Cirocchi R, Rossi M, Parisi A. Indocyanine green fluorescence angiography versus standard intraoperative methods for prevention of anastomotic leak in colorectal surgery: meta-analysis. Br J Surg. 2021; 108:359–372. PMID: 33778848.

24. De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020; 34:53–60. PMID: 30903276.

25. Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Colorectal Dis. 2020; 22:1147–1153. PMID: 32189424.

26. Jafari MD, Pigazzi A, McLemore EC, Mutch MG, Haas E, Rasheid SH, et al. Perfusion Assessment in Left-Sided/Low Anterior Resection (PILLAR III): a randomized, controlled, parallel, multicenter study assessing perfusion outcomes with PINPOINT near-infrared fluorescence imaging in low anterior resection. Dis Colon Rectum. 2021; 64:995–1002. PMID: 33872284.

27. De Robles MS, Young CJ. Triple-staple technique effectively reduces operating time for rectal anastomosis. Ann Coloproctol. 2021; 37:16–20. PMID: 32054240.

28. Tebala GD, Mingoli A, Natili A, Khan AQ, Brachini G. Surgical risk and pathological results of emergency resection in the treatment of acutely obstructing colorectal cancers: a retrospective cohort study. Ann Coloproctol. 2021; 37:21–28. PMID: 32178504.

29. Meijer RP, Faber RA, Bijlstra OD, Braak JP, Meershoek-Klein Kranenbarg E, Putter H, et al. AVOID; a phase III, randomised controlled trial using indocyanine green for the prevention of anastomotic leakage in colorectal surgery. BMJ Open. 2022; 12:e051144.

30. Iguchi K, Watanabe J, Suwa Y, Chida K, Atsumi Y, Numata M, et al. The usefulness of indocyanine green fluorescence imaging for intestinal perfusion assessment of intracorporeal anastomosis in laparoscopic colon cancer surgery. Int J Colorectal Dis. 2023; 38:7. PMID: 36625972.

31. Rottoli M, Tanzanu M, Lanci AL, Gentilini L, Boschi L, Poggioli G. Mesenteric lengthening during pouch surgery: technique and outcomes in a tertiary centre. Updates Surg. 2021; 73:581–586. PMID: 33492620.

32. Freund MR, Kent I, Agarwal S, Wexner SD. Use of indocyanine green fluorescence angiography during ileal J-pouch surgery requiring lengthening maneuvers. Tech Coloproctol. 2022; 26:181–186. PMID: 35091791.

33. Hardy NP, Dalli J, Khan MF, Andrejevic P, Neary PM, Cahill RA. Inter-user variation in the interpretation of near infrared perfusion imaging using indocyanine green in colorectal surgery. Surg Endosc. 2021; 35:7074–7081. PMID: 33398567.

34. Faber RA, Tange FP, Galema HA, Zwaan TC, Holman FA, Peeters KC, et al. Quantification of indocyanine green near-infrared fluorescence bowel perfusion assessment in colorectal surgery. Surg Endosc. 2023; 37:6824–6833. PMID: 37286750.

35. Soares AS, Bano S, Clancy NT, Stoyanov D, Lovat LB, Chand M. Multisensor perfusion assessment cohort study: Preliminary evidence toward a standardized assessment of indocyanine green fluorescence in colorectal surgery. Surgery. 2022; 172:69–73. PMID: 35168814.

36. Skrovina M, Bencurik V, Martinek L, Machackova M, Bartos J, Andel P, et al. The significance of intraoperative fluorescence angiography in miniinvasive low rectal resections. Wideochir Inne Tech Maloinwazyjne. 2020; 15:43–48. PMID: 32117485.

37. Yanagita T, Hara M, Osaga S, Nakai N, Maeda Y, Shiga K, et al. Efficacy of intraoperative ICG fluorescence imaging evaluation for preventing anastomotic leakage after left-sided colon or rectal cancer surgery: a propensity score-matched analysis. Surg Endosc. 2021; 35:2373–2385. PMID: 33495878.

38. Castagneto-Gissey L, Iodice A, Urciuoli P, Pontone S, Salvati B, Casella G. Novel modality of endoluminal anastomotic integrity assessment with fluoroangiography after left-sided colorectal resections. World J Surg. 2023; 47:1303–1309. PMID: 36694037.

39. Lauricella S, Peyser D, Carrano FM, Sylla P. Intraluminal anastomotic assessment using indocyanine green near-infrared imaging for left-sided colonic and rectal resections: a systematic review. J Gastrointest Surg. 2023; 27:615–625. PMID: 36604377.

40. Chand M, Keller DS, Joshi HM, Devoto L, Rodriguez-Justo M, Cohen R. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech Coloproctol. 2018; 22:271–277. PMID: 29551004.

41. Ahn HM, Son GM, Lee IY, Shin DH, Kim TK, Park SB, et al. Optimal ICG dosage of preoperative colonoscopic tattooing for fluorescence-guided laparoscopic colorectal surgery. Surg Endosc. 2022; 36:1152–1163. PMID: 33638107.

42. Ribero D, Mento F, Sega V, Lo Conte D, Mellano A, Spinoglio G. ICG-guided lymphadenectomy during surgery for colon and rectal cancer-interim analysis of the GREENLIGHT Trial. Biomedicines. 2022; 10:541. PMID: 35327344.

43. Wan J, Wang S, Yan B, Tang Y, Zheng J, Ji H, et al. Indocyanine green for radical lymph node dissection in patients with sigmoid and rectal cancer: randomized clinical trial. BJS Open. 2022; 6:zrac151. PMID: 36515673.

44. Ho MF, Futaba K, Mak TW, Ng SS. Personalized laparoscopic resection of colon cancer with the use of indocyanine green lymph node mapping: technical and clinical outcomes. Asian J Endosc Surg. 2022; 15:563–568. PMID: 35261162.

45. Ushijima H, Kawamura J, Ueda K, Yane Y, Yoshioka Y, Daito K, et al. Visualization of lymphatic flow in laparoscopic colon cancer surgery using indocyanine green fluorescence imaging. Sci Rep. 2020; 10:14274. PMID: 32868829.

46. Kinoshita H, Kawada K, Itatani Y, Okamura R, Oshima N, Okada T, et al. Timing of real-time indocyanine green fluorescence visualization for lymph node dissection during laparoscopic colon cancer surgery. Langenbecks Arch Surg. 2023; 408:38. PMID: 36650252.

47. Lucas K, Melling N, Giannou AD, Reeh M, Mann O, Hackert T, et al. Lymphatic mapping in colon cancer depending on injection time and tracing agent: a systematic review and meta-analysis of prospective designed studies. Cancers (Basel). 2023; 15:3196. PMID: 37370806.

48. Ankersmit M, Bonjer HJ, Hannink G, Schoonmade LJ, van der Pas MH, Meijerink WJ. Near-infrared fluorescence imaging for sentinel lymph node identification in colon cancer: a prospective single-center study and systematic review with meta-analysis. Tech Coloproctol. 2019; 23:1113–1126. PMID: 31741099.

49. Villegas-Tovar E, Jimenez-Lillo J, Jimenez-Valerio V, Diaz-Giron-Gidi A, Faes-Petersen R, Otero-Piñeiro A, et al. Performance of Indocyanine green for sentinel lymph node mapping and lymph node metastasis in colorectal cancer: a diagnostic test accuracy meta-analysis. Surg Endosc. 2020; 34:1035–1047. PMID: 31754853.

50. Burghgraef TA, Zweep AL, Sikkenk DJ, van der Pas MH, Verheijen PM, Consten EC. In vivo sentinel lymph node identification using fluorescent tracer imaging in colon cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021; 158:103149. PMID: 33450679.

51. Kim MC, Oh JH. Lateral pelvic lymph node dissection after neoadjuvant chemoradiotherapy in patients with rectal cancer: a single-center experience and literature review. Ann Coloproctol. 2021; 37:382–394. PMID: 34961302.

52. Mahendran B, Balasubramanya S, Sebastiani S, Smolarek S. Extended lymphadenectomy in locally advanced rectal cancers: a systematic review. Ann Coloproctol. 2022; 38:3–12. PMID: 34788526.

53. Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, et al. Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol. 2019; 37:33–43. PMID: 30403572.

54. Lim BL, Park IJ, Kim YI, Kim CW, Lee JL, Yoon YS, et al. Difference in prognostic impact of lateral pelvic lymph node metastasis between pre- and post-neoadjuvant chemoradiotherapy in rectal cancer patients. Ann Surg Treat Res. 2023; 104:205–213. PMID: 37051159.

55. Zhou SC, Tian YT, Wang XW, Zhao CD, Ma S, Jiang J, et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol. 2019; 25:4502–4511. PMID: 31496628.

56. Dai JY, Han ZJ, Wang JD, Liu BS, Liu JY, Wang YC. Short-term outcomes of near-infrared imaging using indocyanine green in laparoscopic lateral pelvic lymph node dissection for middle-lower rectal cancer: a propensity score-matched cohort analysis. Front Med (Lausanne). 2022; 9:1039928. PMID: 36438036.

57. Kim HJ, Choi GS, Park JS, Park SY, Cho SH, Seo AN, et al. S122: impact of fluorescence and 3D images to completeness of lateral pelvic node dissection. Surg Endosc. 2020; 34:469–476. PMID: 31139999.

58. Watanabe J, Ohya H, Sakai J, Suwa Y, Goto K, Nakagawa K, et al. Long-term outcomes of indocyanine green fluorescence imaging-guided laparoscopic lateral pelvic lymph node dissection for clinical stage II/III middle-lower rectal cancer: a propensity score-matched cohort study. Tech Coloproctol. 2023; 27:759–767. PMID: 36773172.

59. Su H, Xu Z, Bao M, Luo S, Liang J, Pei W, et al. Lateral pelvic sentinel lymph node biopsy using indocyanine green fluorescence navigation: can it be a powerful supplement tool for predicting the status of lateral pelvic lymph nodes in advanced lower rectal cancer. Surg Endosc. 2023; 37:4088–4096. PMID: 36997652.

60. Yasui M, Ohue M, Noura S, Miyoshi N, Takahashi Y, Matsuda C, et al. Exploratory analysis of lateral pelvic sentinel lymph node status for optimal management of laparoscopic lateral lymph node dissection in advanced lower rectal cancer without suspected lateral lymph node metastasis. BMC Cancer. 2021; 21:911. PMID: 34380428.

61. Satou S, Ishizawa T, Masuda K, Kaneko J, Aoki T, Sakamoto Y, et al. Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J Gastroenterol. 2013; 48:1136–1143. PMID: 23179608.

62. Barabino G, Klein JP, Porcheron J, Grichine A, Coll JL, Cottier M. Intraoperative near-infrared fluorescence imaging using indocyanine green in colorectal carcinomatosis surgery: proof of concept. Eur J Surg Oncol. 2016; 42:1931–1937. PMID: 27378159.

63. Liberale G, Vankerckhove S, Caldon MG, Ahmed B, Moreau M, Nakadi IE, et al. Fluorescence imaging after indocyanine green injection for detection of peritoneal metastases in patients undergoing cytoreductive surgery for peritoneal carcinomatosis from colorectal cancer: a pilot study. Ann Surg. 2016; 264:1110–1115. PMID: 27828822.

64. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000; 65:271–284. PMID: 10699287.

65. Maeda H. The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci. 2013; 104:779–789. PMID: 23495730.

66. Liberale G, Bourgeois P, Larsimont D, Moreau M, Donckier V, Ishizawa T. Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: a systematic review. Eur J Surg Oncol. 2017; 43:1656–1667. PMID: 28579357.

67. Kim YJ, Kim CH. Treatment for peritoneal metastasis of patients with colorectal cancer. Ann Coloproctol. 2021; 37:425–433. PMID: 34961304.

68. Lieto E, Auricchio A, Cardella F, Mabilia A, Basile N, Castellano P, et al. Fluorescence-guided surgery in the combined treatment of peritoneal carcinomatosis from colorectal cancer: preliminary results and considerations. World J Surg. 2018; 42:1154–1160. PMID: 28929277.

69. González-Abós C, Selva AB, de Lacy FB, Valverde S, A lmenara R, Lacy AM. Quantitative indocyanine green fluorescence imaging assessment for nonmucinous peritoneal metastases: preliminary results of the ICCP Study. Dis Colon Rectum. 2022; 65:314–321. PMID: 34775406.

70. Baiocchi GL, Gheza F, Molfino S, Arru L, Vaira M, Giacopuzzi S. Indocyanine green fluorescence-guided intraoperative detection of peritoneal carcinomatosis: systematic review. BMC Surg. 2020; 20:158. PMID: 32680492.

71. Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006; 141:460–467. PMID: 16702517.
72. Park SH, Shin JK, Lee WY, Yun SH, Cho YB, Huh JW, et al. Clinical outcomes of neoadjuvant chemotherapy in colorectal cancer patients with synchronous resectable liver metastasis: a propensity score matching analysis. Ann Coloproctol. 2021; 37:244–252. PMID: 34182620.

73. Liu W, Zhang ZY, Yin SS, Yan K, Xing BC. Contrast-enhanced intraoperative ultrasound improved sensitivity and positive predictive value in colorectal liver metastasis: a systematic review and meta-analysis. Ann Surg Oncol. 2021; 28:3763–3773. PMID: 33247361.

74. Peloso A, Franchi E, Canepa MC, Barbieri L, Briani L, Ferrario J, et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB (Oxford). 2013; 15:928–934. PMID: 23458105.

75. He K, Hong X, Chi C, Cai C, An Y, Li P, et al. Efficacy of near-infrared fluorescence-guided hepatectomy for the detection of colorectal liver metastases: a randomized controlled trial. J Am Coll Surg. 2022; 234:130–137. PMID: 35213433.

76. ASGE Technology Committee. Kethu SR, Banerjee S, Desilets D, Diehl DL, Farraye FA, et al. Endoscopic tattooing. Gastrointest Endosc. 2010; 72:681–685. PMID: 20883844.

77. Miyoshi N, Ohue M, Noura S, Yano M, Sasaki Y, Kishi K, et al. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg Endosc. 2009; 23:347–351. PMID: 18443867.

78. Watanabe M, Tsunoda A, Narita K, Kusano M, Miwa M. Colonic tattooing using fluorescence imaging with light-emitting diode-activated indocyanine green: a feasibility study. Surg Today. 2009; 39:214–218. PMID: 19280280.

79. Konstantinidis MK, Ioannidis A, Vassiliu P, Arkadopoulos N, Papanikolaou IS, Stavridis K, et al. Preoperative tumor marking with indocyanine green (ICG) prior to minimally invasive colorectal cancer: a systematic review of current literature. Front Surg. 2023; 10:1258343. PMID: 37638121.

80. Noura S, Ohue M, Seki Y, Tanaka K, Motoori M, Kishi K, et al. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann Surg Oncol. 2010; 17:144–151. PMID: 19774415.

Fig. 1
Indocyanine green-fluorescence image (ICG-FI) use for anastomotic safety. Sigmoid colon image under standard white light (A), ICG-FI with red inversion mode (B), time-fluorescence curve of ICG angiography for quantitative perfusion analysis, during laparoscopic low anterior resection for rectal cancer patients. (C) Fluorescent intensity curves have a common transitional area within 4–5 cm as an optimal zone to analyze the fluorescence images (D). Adapted from Son et al. [2] and Ahn et al. [8], according to the Creative Commons License.
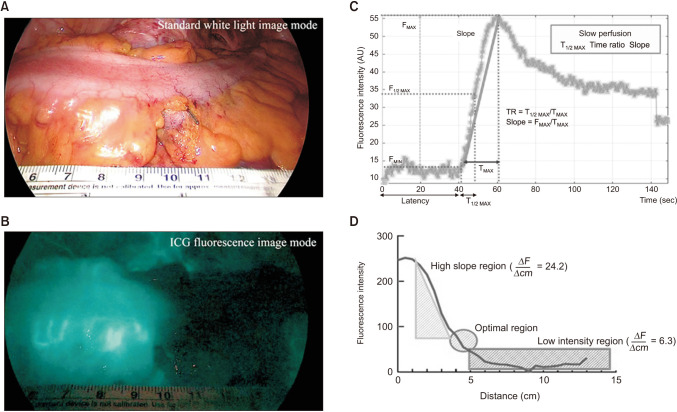
Fig. 2
Fluorescence lymph node mapping using 3 different NIR imaging systems. (A–C) Stryker, 1588 AIM camera system; (D–F) Karl Storz, IMAGE1 S; and (G–I) Olympus, CLV-S200-IR. (A), (D), and (G) show the mesentery of the colon under white light. Adapted from Ahn et al. [41], according to the Creative Commons License.
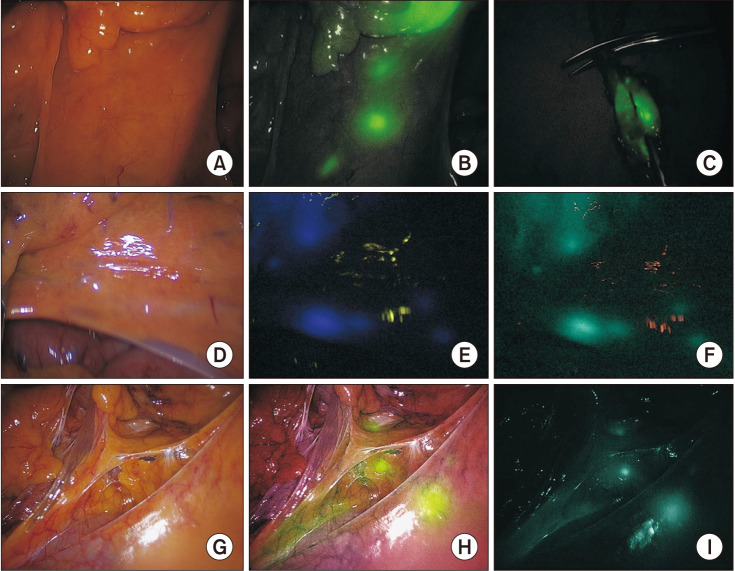
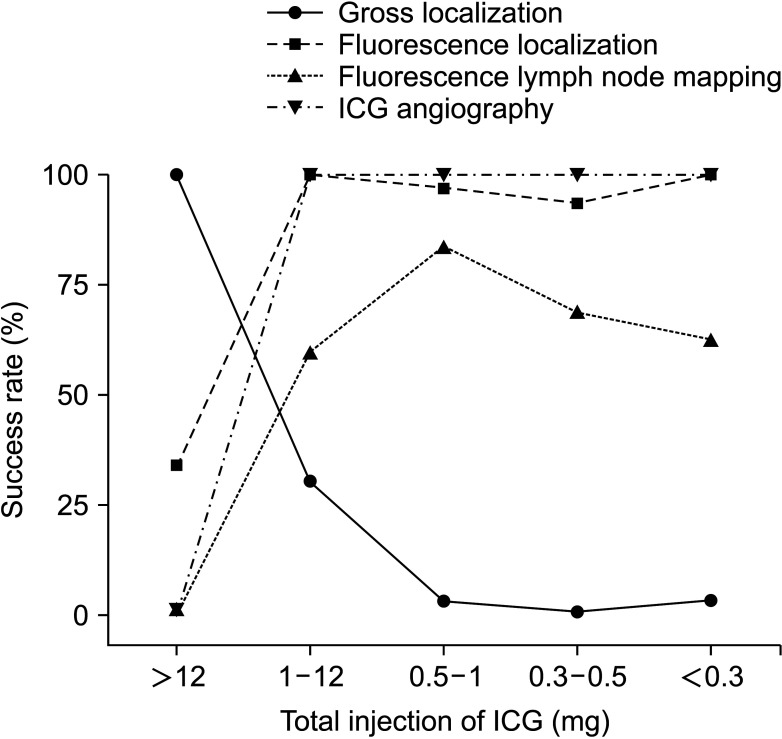
Fig. 3
Optimal indocyanine green (ICG) tattooing protocol. Within a submucosal ICG injection dosage of 0.5–1.0 mg, the protocol was optimized as the highest success rate of fluorescence lymph node mapping along with tumor localization and ICG angiography during a single surgery. Adapted from Ahn et al. [41], according to the Creative Commons License.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download