INTRODUCTION
Chronic kidney disease (CKD) is very common, with one epidemiological study reporting a prevalence of 8.2% in the adult population in Korea and 13.1% in the United States [
123]. CKD-related metabolic and cardiovascular disease are both considered common problems in patients with CKD who may be diagnosed with malignancy. One cohort study found the prevalence of any malignancy in patients with CKD was approximately 7.8% with the most common site being the colorectum (14.6%) [
4]. In a population-based study, end-stage renal disease (ESRD) did not affect the incidence of malignant risk itself; however, the risk was increased specifically in the colon and other specific sites [
5].
Although oncologic outcomes and treatment plans for colorectal malignancy are an important issue in patients with CKD, randomized controlled studies that investigated colorectal malignancy either enrolled very few or completely excluded CKD patients [
67]. Some small retrospective studies explored the oncologic outcomes of colorectal malignancy in CKD patients. However, several of these studies have limitations such as small sample size, selection bias, and retrospective design. These did not reflect real-world outcomes; therefore, the oncologic outcomes of colorectal malignancy in CKD patients are still underestimated in clinical practice.
This study aimed to evaluate the real-world survival outcomes of colorectal malignancy in CKD patients using health insurance claim data provided by the National Health Insurance Service (NHIS) in Korea.
RESULTS
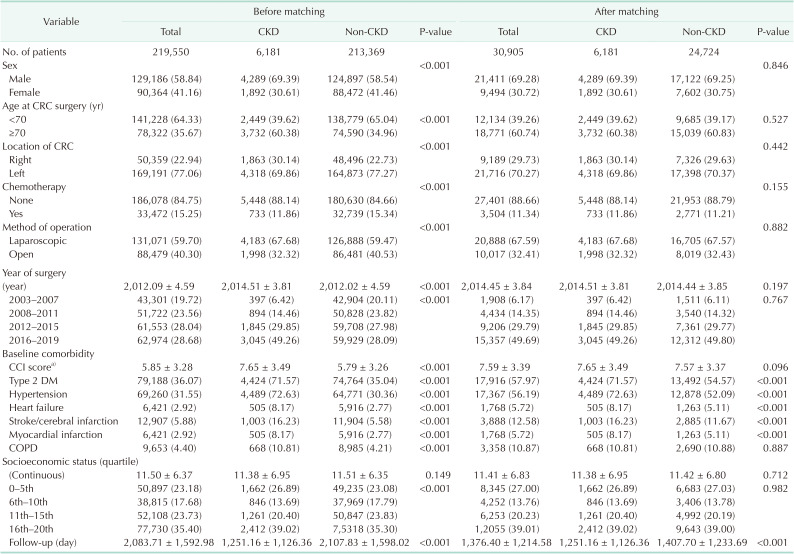
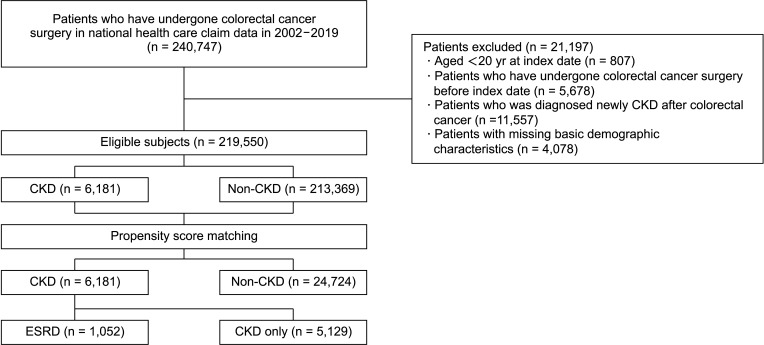
In this study, 219,550 patients received surgery for colorectal cancer (CRC). Among them, 6,181 patients had CKD as an underlying disease and 213,369 patients did not. The characteristics of these patients before and after matching are summarized in
Table 1. The ratio of male patients aged 70 years or older, right colon location, and CCI score were higher in the CKD-CRC group. The ratio of chemotherapy was lower in the CKD-CRC group. Neither factor had any difference after matching.
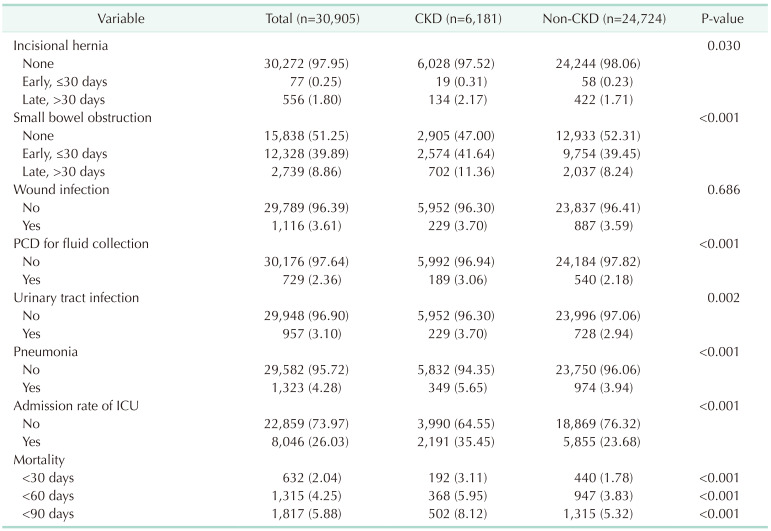
In
Table 2, postoperative morbidity and mortality were compared between the CKD-CRC and non-CKD-CRC groups. The prevalence of each morbidity was significantly higher in the CKD-CRC group. As for the postoperative mortality rate, the 30-day (3.11%
vs. 1.78%, P < 0.001), 60-day (5.95%
vs. 3.83%, P < 0.001), and 90-day mortality rate (8.12%
vs. 5.32%, P = 0.0013) were all significantly higher in the CKD group.
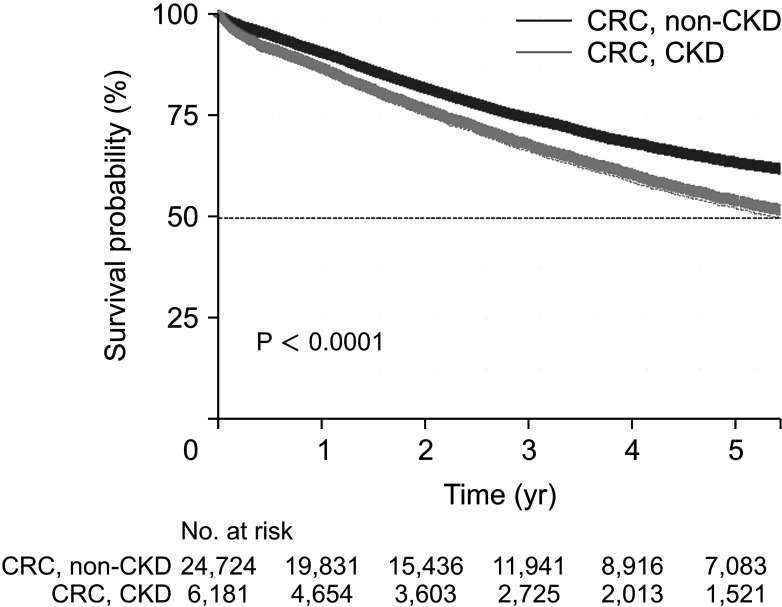
The median survival time was significantly lower in the CKD-CRC group at 5.63 years (interquartile range [IQR], 5.26–5.91 years) than in the non-CKD-CRC group at 8.71 years (IQR, 8.37–8.93 years) (
Fig. 2). In the CKD group, the median survival time was significantly lower among patients who received chemotherapy (4.56 years [IQR, 3.93–5.09 years]
vs. 5.30 years [IQR, 4.73–6.09 years], P = 0.00047) and patients who did not receive chemotherapy (5.79 years [IQR, 5.45–6.10 years]
vs. 8.99 years [IQR, 8.77–9.36 years], P < 0.0001).
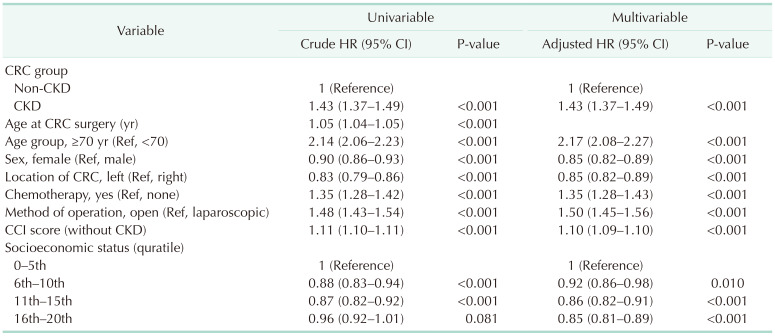
Univariate analysis was conducted on risk factors affecting overall survival. The adjusted result from the multivariate analysis showed an adjusted HR of 1.43 (95% CI, 1.37–1.49; P < 0.001) in the CKD-CRC group, suggesting a significantly higher mortality risk (
Table 3).
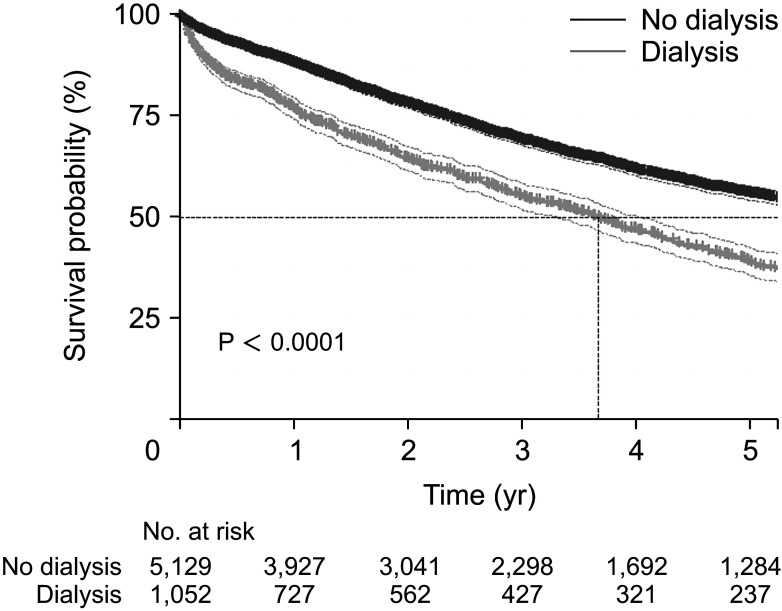
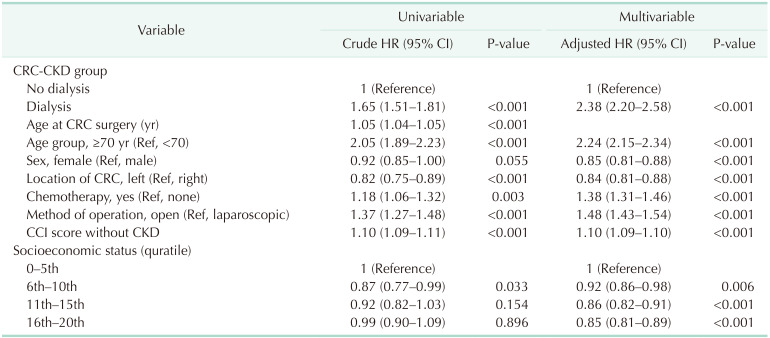
Subgroup analysis was conducted in the CKD-CRC group to determine whether dialysis may have affected risk. When the CKD-CRC group was divided according to whether or not they received dialysis, the median survival time was lower in patients who received dialysis (3.67 years [IQR, 3.32–4.04 years]
vs. 6.23 years [IQR, 5.88–6.68 years], P < 0.0001) (
Fig. 3). Their mortality risk was significantly higher even after adjusting for the risk factors using multivariate analysis compared with that noted in the group who did not receive dialysis (adjusted HR, 2.38; 95% CI, 2.20–2.58; P < 0.001) (
Table 4).
DISCUSSION
In this study, the real-world oncologic outcomes between the CKD-CRC group and the non-CKD-CRC group were examined using a large-scale database. Among 219,550 patients who received surgeries for CRC that met the inclusion criteria between 2002 and 2019, 6181 patients were diagnosed with CKD at the time of operation. The mean follow-up duration was 5.7 years for all patients. After 1:4 propensity matching and adjustment of risk factors, the median survival time was 8.71 years (IQR, 8.37–8.93 years) in the non-CKD-CRC group and 5.63 years (IQR, 5.26–5.91 years) in the CKD-CRC group, indicating that CKD itself is a significant risk factor for survival. Furthermore, the subgroup analysis within the CKD group showed that dialysis is a significant risk factor for survival.
As CKD is excluded from the inclusion criteria in large-scale clinical studies of cancer, there are few studies on the survival of CKD-CRC patients. Therefore, indirect assessment of crude survival in CKD through real-world survival analysis using a nationwide database is necessary. It is widely known that CKD quickly progresses to advanced stage CKD with increasing age [
10] and that increased comorbidities lead to decreased survival. Additionally, due to decreased glomerular filtration rate, patients receive relatively low-dose or no chemotherapy [
11]. These patients are excluded from large-scale clinical studies of patients with CKD, which is the main reason underlying the limited analyses of survival and treatment outcomes in cancer patients with CKD. Recently, a retrospective study reported that was a CKD itself in CKD-CRC patients compared to CRC patients (HR, 1.17; 95% CI, 0.75–1.80; P = 0.49) [
12]. A
post-hoc analysis reported that cancer-related mortality is higher in patients with hypertension (HTN)-CKD than those with HTN (HR, 2.28; 95% CI, 1.12–4.62; P = 0.048) [
13]. In our study, the 5-year survival was 62.69% and 52.75% in the non-CKD-CRC and CKD-CRC group, respectively. After propensity matching, multivariate analysis showed significant decrease in survival from CKD itself (adjusted HR, 1.42; 95% CI, 1.37–1.49; P < 0.001). However, the chemotherapeutic dose needs to be adjusted in patients with CKD and, consequently, the effect may be lower than that of the full dose. Additionally, the drugs available can be limited, and the life expectancy of the patient needs to be considered comprehensively for chemotherapy [
14]. Therefore, compared to the patients without CKD, patients with CKD may have received chemotherapy at a lower rate among those for whom chemotherapy was suitable. However, the effects of chemotherapy can be further adjusted if the patient’s specific stage of CRC could be identified and the actual ratio of patients who had received chemotherapy could be confirmed.
When we adjusted for several confounding factors—namely age, comorbidity, and chemotherapy—which influence mortality in CKD, a lower survival rate was revealed in the CKD-CRC than in the non-CKD-CRC group. This suggests that CKD itself affects the onset or treatment of cancer. According to in vivo experiments, gut motility was reduced in CKD [
15], while dysbiosis and uremic toxins originating from the gut bacterial metabolism increased proinflammatory status and decreased serum concentration of OATP2B1 substrates that have an anticancer effect [
1617]. Additionally, studies on the relationship between the gut microbiome and CRC risk reported that dysbiosis of the microbiome induces chronic inflammation which in turn affects carcinogenesis and tumor progression [
1819]. Therefore, it can be speculated that constant changes in the gut microbiome due to CKD affect the onset and treatment of CRC. However, further studies are required to explore the possible correlation between CKD-induced changes in gut microbiota and the previously reported effects of microbiota on cancer.
It has been reported that all-cause mortality gradually increases according to the CKD severity and that the 5-year survival of ESRD patients is 41%–48% and 44%–60% in Western and Eastern countries, respectively [
1020]. In our study, the crude 5-year survival was lower in the CRC-ESRD patients than in the CRC-CKD patients (adjusted HR, 2.48; 95% CI, 2.29–2.69; P < 0.001). This result is in line with a decreased survival rate according to CKD severity. However, our results show a lower survival rate than that reported for all the ESRD patients combined in a previous study. This is likely due to the limited effects of chemotherapy due to dialysis, in addition to ESRD itself, as well as insufficient performance of chemotherapy due to increased comorbidity.
Our study showed an increased morbidity in the CRC-CKD group, with a mortality rate 1.74 times higher within 30 days postoperatively (3.11
vs. 1.78). This increased postoperative morbidity and mortality is shown by the high 30-day postoperative morbidity for CKD patients who received dialysis compared to CRC-CKD patients. Similarly, it has been reported that CKD patients had a higher morbidity compared to general patients [
21], and those who had dialysis among CKD patients presented with higher morbidity and mortality [
2223]. Other studies have reported a similar increase. However, our study found a low postoperative mortality rate in the CKD patients compared to other studies. Hence, surgery should be implemented more often in the CKD-CRC cases, while scrupulously considering the morbidity of each individual patient.
The strength of our study is that it presents real-world survival rates since it used a nationwide data set. Patients’ chance of survival can be predicted prior to colorectal surgery according to the underlying conditions of CKD patients, which may offer an objective guideline for proposing suitable treatment based on the identified clinical stage.
This study has several limitations. First, it is a retrospective review with limited to access the lymphovascular invasion, tumor budding, tumor deposit, stage of colorectal malignancy, emergency operation, and cause of death all of which can affect findings. Specifically, because we were unable to identify the patients’ colon cancer stage, these results must be carefully interpreted. Secondly, due to the various types of comorbidities, we used CCI as opposed to other scoring systems proven to be associated with survival. As CKD was included as one of the score items, a modified CCI that excluded CKD score was used for the study. However, as it has not been verified that the modified CCI can truly reflect the comorbidity that predicts patients’ survival, the objective probability cannot be guaranteed, and will only offer an indirect assessment. Finally, we were also unable to identify CKD status, and thus clarify which patients may have had different morbidity and mortality risks. CKD status has been related to comorbidity, immunity, and tumorigeneses. To overcome these limitations, various factors that can affect survival were adjusted to implement propensity matching for the entire group. However, propensity matching was not performed for dialysis patients, and only subgroup analysis was conducted within the group. This was due to the small number of dialysis patients in our sample, which restricted the classification of groups during matching. Hence, the analysis of dialysis patients is limited in our study.
In conclusion, we investigated the real-world survival of CKD-CRC patients and determined CKD and ESRD to be risk factors for survival. Prevention of CKD-to-ESRD progression might be one of the strategies to increase postoperative survival.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download