Abstract
Recent advancements in endoscopic procedures have resulted in a growing diagnosis of early colorectal cancer (CRC) cases, where classical en bloc lymph node (LN) dissection is not performed and treatment is terminated with the removal of the main cancer lesion by endoscopy without pathologic LN staging. Although many studies report noninferior outcomes of endoscopic resection in comparison to surgical resection, a cautious approach to completing treatment with endoscopic resection alone is recommended because LN metastases may be present even in early-stage CRC. In most countries, including the United States, Europe, and South Korea, the guidelines for additional surgery after endoscopic resection are very similar. If LN metastasis is suspected, even in T1 stage or lower lesions, further surgery is an essential treatment modality, but confirmation of the presence of LN metastasis is perhaps the most difficult part of this process. Another paradoxical recent trend is the expansion of more extensive and complete surgical lymphadenectomy for CRC. The success rate of surgery has improved dramatically over the past decade with the introduction of surgical devices and minimally invasive surgery, and the associated risks have been significantly reduced. While the burden of surgery on patients is understandable, the indications for surgery in early colon cancer need to be carefully reviewed to improve cure rates. In this process, we believe that an integrated decision-making process with surgeons, radiologists, and pathologists, in addition to the opinions of endoscopists, will be an important process to improve the cure rate.
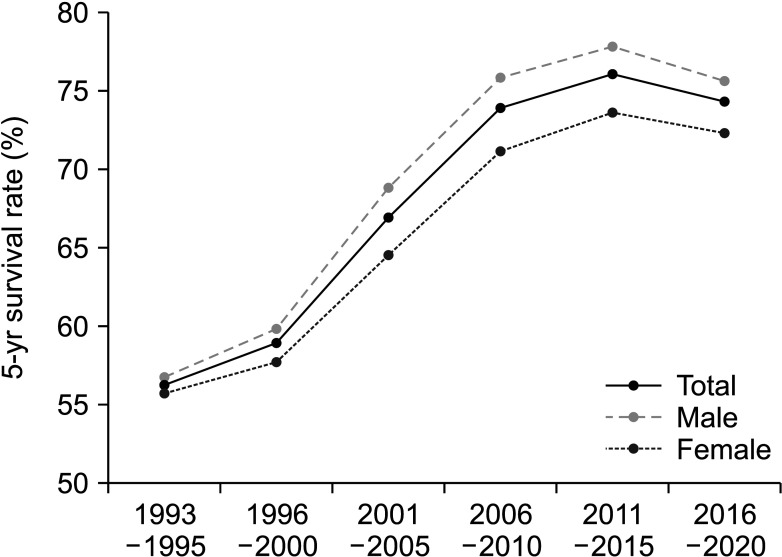
Colorectal cancer (CRC) is a significant cause of cancer-related death worldwide. In Korea, CRC still occupied third position among all types of cancers, despite its decreasing trend over the previous 10 years [1]. A notable improvement in the survival rate was observed over the past 20 years, as indicated by the statistics report of the National Cancer Information Center in 2019 (Fig. 1). The survival rate increased from 56.2% to 74.3% across all stages [2]. This improvement in survival rates is due to a combination of factors including expanded national screening programs, propagation of concepts for early diagnosis and treatment, advances in surgical techniques, and the development of anticancer drugs [3].
Cancer staging is a priority for treatment planning, and in the process, identifying the lymph node (LN) metastasis in colon cancer is a basic diagnostic. Additionally, it is a therapeutic step in optimizing patient outcomes. Nodal metastasis is a key factor in determining colon cancer prognosis based on the American Joint Committee on Cancer (AJCC) staging system [45].
Despite the high quality of radiologic studies, such as CT, ultrasound, and MRI, which are performed for clinical staging, pathologic staging with microscopic examination of LN collected during surgery is regarded as the most accurate examination. However, recent advancements in endoscopic procedures have resulted in a growing diagnosis of early CRC cases, where classical en bloc LN dissection is not performed and treatment is terminated with the removal of the main cancer lesion by endoscopy without pathologic LN staging. This trend is expected to continue to expand owing to its significant advantages, including tumor removal without the need for anesthesia, hospitalization, and open surgery.
Additionally, the proportion of mucosal and submucosal cancers is increasing as the rate of cancer detected before the appearance of symptoms through national screening increases. Endoscopic resection for early-stage CRC is based on the assumption that early-stage CRC has a low risk of LN metastasis. If LN metastases occur, the CRC stage is upscaled to stage III; therefore, it is crucial to identify the presence of LN metastasis because it is associated with a poor prognosis and is subject to adjuvant treatment [6]. From this perspective, colorectal surgeons have always been cognizant of the standard surgical guidelines that recommend the combined resection of the colon and the primary blood supply vessel, as well as LN dissection in the surrounding area.
Many studies, including meta-analyses, have reported that endoscopic resection does not show inferior clinical outcomes compared to surgical resection. Therefore, the significance and indications of standard surgical lymphadenectomy for early colon cancer should be reconsidered.
This review was designed to explore the significance of LN metastases in CRC and to clarify the criteria for surgeon preparation for standard lymphadenectomy.
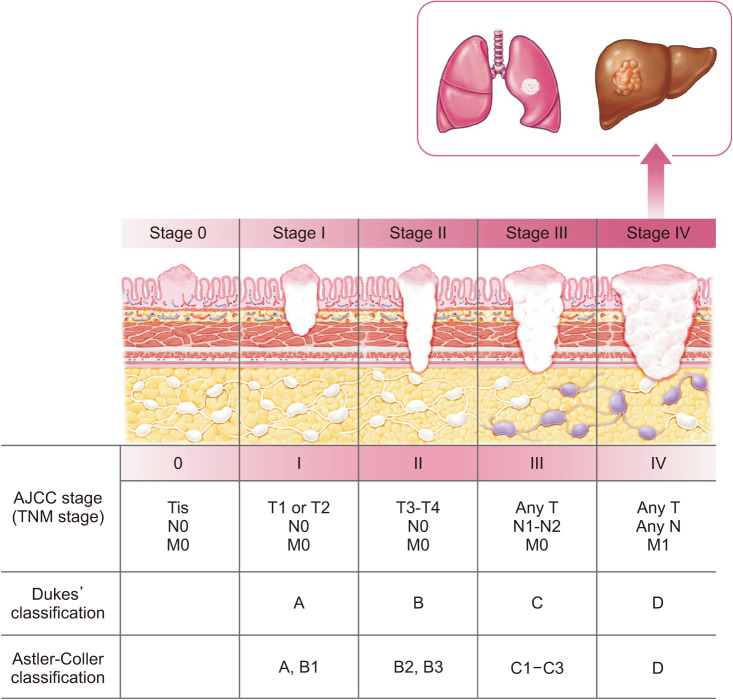
CRC staging was initially proposed by Dukes in 1932 (Fig. 2) [7], which is based on the pathological assessment of tumor spread and LN metastasis specifically in rectal cancer. Dukes classified the disease into 3 stages: tumors confined to the rectum, tumors outside the rectal wall, and those with metastases to the surrounding LNs. These groupings were established based on Dr. Dukes’ observations and the classification of many rectal tumor specimens during his work at St. Marks Hospital in London, which had a strong focus on perineal surgery [8].
Further subclassification and modification of staging were performed by Astler and Coller [9] in 1954 (Fig. 2). They proposed splitting stage A into stages A and B1. Only superficial tumors limited to the mucosa were included in stage A, and those infiltrating the submucosa but not crossing the muscularis propria were included in stage B1. Ex-stage B becomes B2. No metastases were observed in stages A, B1, or B2. Cases with positive LNs were included in stages C1 and C2, corresponding to stages B1 and B2, respectively, with associated LN metastases. In addition to this division, Turnbull proposed stage D for tumors with distant metastasis to other organs, and the 3 main factors determining the stage were not significantly changed despite changes in the mainstream staging system over the decades.
Since 1977, TNM staging by the AJCC has been established internationally and the 8th edition was released in 2016 with continuous refinement (Fig. 2) [10]. In the 8th edition, the AJCC expanded the use of nonanatomic prognostic factors and biomarkers in assigning prognostic stage groups. It is believed that the AJCC will continue to change and develop minor modifications that will lead to better clinical decision-making. A section on the currently available risk assessment models is included in the Disease Site chapter for a few pilot sites in the 8th edition.
Endoscopic resection is one of the most significant advances in the treatment of CRC. Endoscopic resection of colon neoplasms is also a topical issue in global healthcare within the current screening programs for CRC and reduction of cancer-related mortality [111213]. Before the introduction of endoscopic resection, surgical removal of diseased bowel with lymphadenectomy was the preferred treatment for all stages of CRC, including early-stage CRC.
Endoscopic resection for early-stage CRC is now well established, with its safety confirmed by cumulative clinical data, meta-analyses, and systemic reviews reporting data from multiple institutions. The increased use of endoscopic resection is also supported by advances in endoscopic instruments, such as variable-sized transparent caps, submucosal injectants, knives, hooks, and more [14]. Depending on the depth of the tumor, endoscopic mucosal resection (EMR) may be attempted, or endoscopic submucosal dissection (ESD) may be performed. The criteria for these procedures are well documented in textbooks and are commonly practiced worldwide. The basic principle of EMR involves elevating the tumor using a saline injection, encircling the affected mucosa using a snare device, and excising it with electrocautery. Regarding ESD, after submucosal injection, the mucosal margin is incised, and dissection along the submucosa is continued with caution to mitigate unwanted complications such as perforation or bleeding.
The European Society of Gastrointestinal Endoscopy has published clinical guidelines for colonic polypectomy and EMR [1516]. According to their guidelines, endoscopic procedures should be approached cautiously in cases of lesion size exceeding 40 mm, ileocecal valve location, previously failed resections, and lesion size, shape, as well as location, which are associated with incomplete resection or tumor recurrence. They suggested that surgical resection or ESD may be appropriate alternatives for high-risk lesions, although EMR is an effective and safe procedure, specifically when suggestive of invasive disease. Because the perforation rate associated with colorectal ESD is as high as 1.4%–20.4%, the selection for ESD has been challenging until now.
The United States Multi-Society Task Force released recommendations for endoscopic removal of CRC in 2020 [17]. They recommended EMR for nonpedunculated lesions of >20 mm and referral for surgery in cases suggestive of deep submucosal tumors. Furthermore, they mentioned endoscopic full-thickness resection in the colon and rectum, which was recently focused on as a substitute for the surgical removal of submucosal lesions. The development of full-thickness resection is based on recent advances in new instruments and expanded usage of traditional tools, as well as advances in endoscopist skills and clinical experience. It is still too early to confirm the safety and prognosis of this method and further accumulation of clinical results is required.
The Korean Society of Gastroenterology released the Clinical Practice Guidelines for the Endoscopic Resection of Early Gastrointestinal Cancer in 2020 [1218]. Because recurrence and LN metastasis of submucosal CRC after EMR are more suspected, additional surgery is recommended when the histological findings after endoscopic resection suggest a high risk of LN metastasis, such as poor histologic types (poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma).
As aforementioned, the guidelines for EMRs worldwide are not very different. Because most aim to expand the indications for endoscopically resectable lesions and provide stable follow-up, future trends in Korea will likely continue to change steadily.
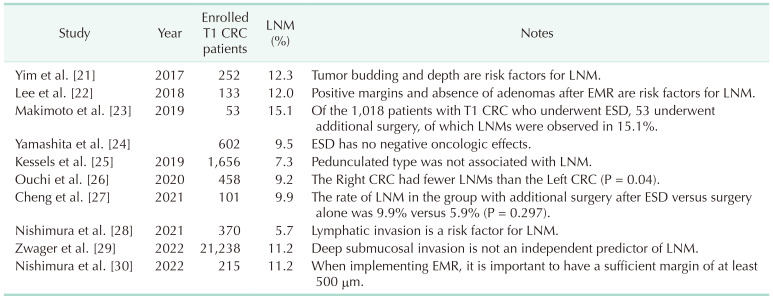
Clinical staging based on CT is usually the first step in planning the endoscopic resection of a suspected malignant colorectal tumor. Endoscopic resection was attempted when clinical staging results showed no evidence of LN enlargement around the tumor or systemic metastasis. This is based on the premise that the endoscopic resection of malignant tumors is based on clinical staging. It is not uncommon for clinicians to encounter a case where the clinical stage is thought to be N0 at the time of attempted endoscopic resection, but when surgical resection is subsequently performed to confirm the histologic stage, the final pathologic stage is N1 or higher (Table 1) [192021222324252627282930].
In CT images, LNs with a diameter greater than 10 mm are usually regarded as malignant, but other imaging features have also been examined, such as border irregularities, nodal texture heterogeneity, and nodal shape [3132]. Unfortunately, the reported accuracy of CT for detecting LN metastasis has not been satisfactory. To provide more refined predictability, radiologists attempted to define the accuracy of LN metastasis.
Li et al. [33] reported LN features on CT scans of patients with T1 cancer who underwent surgery, which revealed that tumor necrosis, irregular outer border, and heterogeneous enhancement were significantly related to nodal metastasis. Therefore, it would help make decisions regarding definitive surgery for early colon cancer.
PET, which is less useful for minute malignant lesions, is not helpful for LN staging in early colon cancer. To date, CT is considered the most useful radiologic tool for predicting LN metastasis, despite its unsatisfactory accuracy.
Due to the limitations of radiological examination of LN metastasis, endoscopists have focused on the predictive value of endoscopic findings and microscopic features of the tumor itself.
Bianco et al. [34] reported that a tumor size of >10 mm, sessile type tumor, submucosal 3 lesion, insufficient margin in univariate analysis, borderline margin, and lymphovascular invasion in multivariate analysis have significant predictive power for LN metastasis. Accordingly, the accuracy of radiological diagnosis combined with endoscopic findings is currently the most meaningful method for predicting LN metastasis.
Traditional CRC surgery is based on the principle of en bloc resection, which involves simultaneous ligation of the large intestine where the cancer is located and the major blood vessels to that location and simultaneous resection of most of the soft tissue, including the LNs around the named arteries. The technique proposed by Hohenberger et al. [35] in 2009, namely, complete mesocolic excision (CME), a sequel to the concept of total mesorectal excision in rectal cancer, was introduced and rapidly accepted to reduce the incidence of local recurrence and increase survival in patients with colon cancer [35]. In cases of right-sided colon cancer, dissection is achieved along the anatomical planes (embryonic planes), followed by ligation of the colonic supplying vessels to the right of the superior mesenteric vein and cleaning of the surrounding lymphoadipose tissue.
D3 lymphadenectomy was first proposed in the Japanese guidelines for the treatment of colon and rectal cancer in 1977 [36]. This concept has been established as the surgical standard in Japan, Korea, China, etc ever since. It includes the complete removal of the paracolic, intermediate, and central LNs [37]. This requires further exposure and skeletalization of the major arterial trunk. These 2 concepts are not different, but their points of emphasis are different. Whereas the Western concept of CME focuses on the anatomical concept of resection along the embryological layer, the Japanese concept focuses on the exact grouping and location of the LNs being removed. Karachun et al. [3738] reported that the final result of surgery for the difference between the 2 concepts is the length of the resected bowel, based on a report by West et al. [39] in 2012, which is not a widely accepted theory.
The survival gains of these surgical interventions led surgeons to enthusiastically pursue CME or D3 dissection, and it has been thought to be the gold standard [40]. Recently, however, questions have been raised regarding the biased analyses of these results, and other types of outcomes have been reported, particularly in early colon cancer. In addition, the significance of massive dissection has been questioned because a series of studies showed no difference in survival and disease-free survival between EMR and ESD patients without lymphadenectomy and those who underwent surgical resection [414243444546474849].
Bae et al. [50] reported that the overall outcome of T1 CRC depends on the treatment method. They reported no differences in survival and recurrence rates during a 42-month median follow-up period between patients who underwent endoscopic resection only, endoscopic resection first, surgery later, and surgery first. However, as noted in their discussion, there was a selection bias in determining treatment, which is the most important issue for LN staging.
Recently, Nishizawa et al. [51] released a systematic review of the outcomes of ESD for colorectal epithelial neoplasms. They reviewed 14 articles and reported an average 5-year survival rate of 99.4% and a local recurrence rate of 1.6%. In their results, a local recurrence rate of 3.8–14.5 and a distant metastasis rate of 0%–6.3% were significantly associated with incomplete resection, deep submucosal invasion or vascular and lymphatic invasion, and poor histology. This analysis suggests that surgery should be considered in early CRC, even though statistics show that recurrence rates after endoscopic resection are not higher than those after surgical resection.
Given that the survival rate after endoscopic resection alone is not inferior to that after surgical resection, the value of surgical resection for early colon cancer should be reconsidered. As mentioned earlier, the fundamental difference between EMR and surgical resection is full-thickness removal of the bowel and lymphadenectomy. If there is no difference in outcome between the 2 treatments, it is natural to favor simpler, lower-risk, and less inconvenient treatment for the patient. It is necessary to ensure that the patient is not penalized by an incorrect treatment policy that ignores findings that may suggest recurrence. It is time to detail new guidelines for selecting the patients for surgical resection and the extent of surgery by scaling various information from the EMR [52].
Endoscopic surgery is an excellent treatment option for CRC. As the number of people eligible for colonoscopy increases due to the gradual expansion of screening programs, the importance of diagnosing and treating cancer in its early stages is well understood by the public. As life expectancy is increasing worldwide the incidence of cancer is increasing rapidly. As long as it is safe, there is no reason not to aggressively treat the aging population, and the growing access to endoscopic therapy is very encouraging [53]. However, because LN metastases in early CRC are not uncommon, surgical treatment should be seriously considered in cases of present risk factors such as insufficient margins, submucosal involvement, lymphatic invasion, etc. In the process of planning the treatment, the surgeons need to decide on the treatment method after sufficient review together with specialists of other fields such as endoscopist, radiologist, and pathologist.
References
1. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–430. PMID: 30913865.

2. Korean Statical Information Service (KOSIS). The 5-year relative survival rates by 24 cancers/age at diagnosis/sex [Internet]. KOSIS;2023. cited 2023 Jan 26. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_117N_A00021&vw_cd=MT_ZTITLE&list_id=F_35&scrId=&seqNo=&lang_mode=ko&obj_var_id=&itm_id=&conn_path=MT_ZTITLE&path=%252FstatisticsList%252FstatisticsListIndex.do.
3. Lee J, Yang IJ, Suh JW, Ahn HM, Oh HK, Kim DW, et al. Predicting stage ypT0-1N0 for nonradical management in patients with middle or low rectal cancer who undergo neoadjuvant chemoradiotherapy: a retrospective cohort study. Ann Surg Treat Res. 2022; 103:32–39. PMID: 35919109.

4. O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004; 96:1420–1425. PMID: 15467030.

5. Kim SH, Ha TK, Kwon SJ. Evaluation of the 7th AJCC TNM staging system in point of lymph node classification. J Gastric Cancer. 2011; 11:94–100. PMID: 22076209.

6. Jeong WK, Shin JW, Baek SK. Oncologic outcomes of early adjuvant chemotherapy initiation in patients with stage III colon cancer. Ann Surg Treat Res. 2015; 89:124–130. PMID: 26366381.

7. Banias L, Jung I, Chiciudean R, Gurzu S. From Dukes-MAC staging system to molecular classification: evolving concepts in colorectal cancer. Int J Mol Sci. 2022; 23:9455. PMID: 36012726.

8. Shampo MA. Dukes and broders: pathologic classifications of cancer of the rectum. Urogynecology. 2001; 7:5–7.
9. Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954; 139:846–852. PMID: 13159135.

10. Kattan MW, Hess KR, Amin MB, Lu Y, Moons KG, Gershenwald JE, et al. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin. 2016; 66:370–374. PMID: 26784705.

11. Yang DH, Luvsandagva B, Tran QT, Fauzi A, Piyachaturawat P, Soe T, et al. Colonoscopic polypectomy preferences of asian endoscopists: results of a survey-based study. Gut Liver. 2021; 15:391–400. PMID: 32839364.

12. Park CH, Yang DH, Kim JW, Kim JH, Kim JH, Min YW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc. 2020; 53:142–166. PMID: 32252507.

14. Hayat M, Azeem N, Bilal M. Colon polypectomy with endoscopic submucosal dissection and endoscopic full-thickness resection. Gastrointest Endosc Clin N Am. 2022; 32:277–298. PMID: 35361336.

15. Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017; 49:270–297. PMID: 28212588.

16. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015; 47:829–854. PMID: 26317585.

17. Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, et al. Endoscopic removal of colorectal lesions: recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2020; 115:435–464. PMID: 32058340.

18. Cubiella J, Marzo-Castillejo M, Mascort-Roca JJ, Amador-Romero FJ, Bellas-Beceiro B, Clofent-Vilaplana J, et al. Clinical practice guideline. Diagnosis and prevention of colorectal cancer. 2018 Update. Gastroenterol Hepatol. 2018; 41:585–596. PMID: 30245076.

19. Ha RK, Han KS, Sohn DK, Kim BC, Hong CW, Chang HJ, et al. Histopathologic risk factors for lymph node metastasis in patients with T1 colorectal cancer. Ann Surg Treat Res. 2017; 93:266–271. PMID: 29184880.

20. Paik JH, Ryu CG, Hwang DY. Risk factors of recurrence in TNM stage I colorectal cancer. Ann Surg Treat Res. 2023; 104:281–287. PMID: 37179701.

21. Yim K, Won DD, Lee IK, Oh ST, Jung ES, Lee SH. Novel predictors for lymph node metastasis in submucosal invasive colorectal carcinoma. World J Gastroenterol. 2017; 23:5936–5944. PMID: 28932085.

22. Lee SJ, Kim A, Kim YK, Park WY, Kim HS, Jo HJ, et al. The significance of tumor budding in T1 colorectal carcinoma: the most reliable predictor of lymph node metastasis especially in endoscopically resected T1 colorectal carcinoma. Hum Pathol. 2018; 78:8–17. PMID: 29447923.

23. Makimoto S, Takami T, Hatano K, Kataoka N, Yamaguchi T, Tomita M, et al. Additional surgery after endoscopic submucosal dissection for colorectal cancer: a review of 53 cases. Int J Colorectal Dis. 2019; 34:1723–1729. PMID: 31478085.

24. Yamashita K, Oka S, Tanaka S, Nagata S, Hiraga Y, Kuwai T, et al. Preceding endoscopic submucosal dissection for T1 colorectal carcinoma does not affect the prognosis of patients who underwent additional surgery: a large multicenter propensity score-matched analysis. J Gastroenterol. 2019; 54:897–906. PMID: 31104172.

25. Kessels K, Backes Y, Elias SG, van den Blink A, Offerhaus GJ, van Bergeijk JD, et al. Pedunculated morphology of T1 colorectal tumors associates with reduced risk of adverse outcome. Clin Gastroenterol Hepatol. 2019; 17:1112–1120. PMID: 30130623.

26. Ouchi A, Toriyama K, Kinoshita T, Tanaka T, Shimizu Y, Niwa Y, et al. Variations in clinical features and oncologic behaviors of T1 colorectal cancer according to tumor location. Int J Clin Oncol. 2020; 25:1130–1136. PMID: 32124095.

27. Cheng P, Lu Z, Huang F, Zhang M, Chen H, Zheng Z. Does additional laparoscopic-assisted surgery after endoscopic submucosal dissection affect short outcomes in patients with stage T1 colorectal cancer? A propensity score-based analysis. Dig Surg. 2021; 38:198–204. PMID: 33774616.

28. Nishimura T, Oka S, Tanaka S, Asayama N, Nagata S, Tamaru Y, et al. Clinical significance of immunohistochemical lymphovascular evaluation to determine additional surgery after endoscopic submucosal dissection for colorectal T1 carcinoma. Int J Colorectal Dis. 2021; 36:949–958. PMID: 33150491.

29. Zwager LW, Bastiaansen BA, Montazeri NS, Hompes R, Barresi V, Ichimasa K, et al. Deep submucosal invasion is not an independent risk factor for lymph node metastasis in T1 colorectal cancer: a meta-analysis. Gastroenterology. 2022; 163:174–189. PMID: 35436498.

30. Nishimura T, Oka S, Kamigaichi Y, Tamari H, Shimohara Y, Okamoto Y, et al. Vertical tumor margin of endoscopic resection for T1 colorectal carcinoma affects the prognosis of patients undergoing additional surgery. Surg Endosc. 2022; 36:5970–5978. PMID: 35020058.

31. Son GM, Park SB, Kim TU, Park BS, Lee IY, Na JY, et al. Multidisciplinary treatment strategy for early colon cancer: a review. An English version. J Anus Rectum Colon. 2022; 6:203–212. PMID: 36348951.

32. Park IJ. Clinical implication of lateral pelvic lymph node metastasis in rectal cancer treated with neoadjuvant chemoradiotherapy. Ewha Med J. 2022; 45:3–10.

33. Li S, Li Z, Wang L, Wu M, Chen X, He C, et al. CT morphological features for predicting the risk of lymph node metastasis in T1 colorectal cancer. Eur Radiol. 2023; 33:6861–6871. PMID: 37171490.

34. Bianco F, De Franciscis S, Belli A, Falato A, Fusco R, Altomare DF, et al. T1 colon cancer in the era of screening: risk factors and treatment. Tech Coloproctol. 2017; 21:139–147. PMID: 28194568.

35. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation. Technical notes and outcome. Colorectal Dis. 2009; 11:354–365. PMID: 19016817.
36. Adachi Y, Mimori K, Mori M, Maehara Y, Sugimachi K. Morbidity after D2 and D3 gastrectomy for node-positive gastric carcinoma. J Am Coll Surg. 1997; 184:240–244. PMID: 9060918.
37. Karachun A, Petrov A, Panaiotti L, Voschinin Y, Ovchinnikova T. Protocol for a multicentre randomized clinical trial comparing oncological outcomes of D2 versus D3 lymph node dissection in colonic cancer (COLD trial). BJS Open. 2019; 3:288–298. PMID: 31183444.

38. Karachun A, Panaiotti L, Chernikovskiy I, Achkasov S, Gevorkyan Y, Savanovich N, et al. Short-term outcomes of a multicentre randomized clinical trial comparing D2 versus D3 lymph node dissection for colonic cancer (COLD trial). Br J Surg. 2020; 107:499–508. PMID: 31872869.

39. West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, et al. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol. 2012; 30:1763–1769. PMID: 22473170.

40. Kwak HD, Ju JK, Yeom SS, Lee SY, Kim CH, Kim YJ, et al. Is radical surgery for clinical stage I right-sided colon cancer relevant? A retrospective review. Ann Surg Treat Res. 2020; 98:139–145. PMID: 32158734.

41. Bartel MJ, Brahmbhatt BS, Wallace MB. Management of colorectal T1 carcinoma treated by endoscopic resection from the Western perspective. Dig Endosc. 2016; 28:330–341. PMID: 26718885.

42. Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009; 136:832–841. PMID: 19171141.

43. Ricciardi R, Madoff RD, Rothenberger DA, Baxter NN. Population-based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol. 2006; 4:1522–1527. PMID: 16979956.

44. Cooper GS, Xu F, Barnholtz Sloan JS, Koroukian SM, Schluchter MD. Management of malignant colonic polyps: a population-based analysis of colonoscopic polypectomy versus surgery. Cancer. 2012; 118:651–659. PMID: 21751204.

45. Borschitz T, Gockel I, Kiesslich R, Junginger T. Oncological outcome after local excision of rectal carcinomas. Ann Surg Oncol. 2008; 15:3101–3108. PMID: 18719965.

46. Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015; 81:583–595. PMID: 25592748.

47. Ebigbo A, Probst A, Messmann H. Endoscopic treatment of early colorectal cancer: just a competition with surgery? Innov Surg Sci. 2018; 3:39–46. PMID: 31579764.

48. Kiriyama S, Saito Y, Yamamoto S, Soetikno R, Matsuda T, Nakajima T, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy. 2012; 44:1024–1030. PMID: 23012216.

49. Al-Sawat A, Bae JH, Kim HH, Lee CS, Han SR, Lee YS, et al. Short- and long-term outcomes of local excision with adjuvant radiotherapy in high-risk T1 rectal cancer patients. Ann Surg Treat Res. 2022; 102:36–45. PMID: 35071118.

50. Bae HJ, Ju H, Lee HH, Kim J, Lee BI, Lee SH, et al. Long-term outcomes after endoscopic versus surgical resection of T1 colorectal carcinoma. Surg Endosc. 2023; 37:1231–1241. PMID: 36171453.

51. Nishizawa T, Ueda T, Ebinuma H, Toyoshima O, Suzuki H. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms: a systematic review. Cancers (Basel). 2022; 15:239. PMID: 36612232.

52. Tummala S, Kadry S, Nadeem A, Rauf HT, Gul N. An explainable classification method based on complex scaling in histopathology images for lung and colon cancer. Diagnostics (Basel). 2023; 13:1594. PMID: 37174985.

53. Yang IJ, Oh HK, Lee J, Suh JW, Ahn HM, Shin HR, et al. Efficacy of geriatric multidisciplinary oncology clinic in the surgical treatment decision-making process for frail elderly patients with colorectal cancer. Ann Surg Treat Res. 2022; 103:169–175. PMID: 36128034.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download