INTRODUCTION
Secondary functional tricuspid regurgitation (TR) due to annular dilatation is mainly treated with tricuspid annuloplasty (TAP). However, severe TR with complex tricuspid valve (TV) pathologies, such as severe leaflet tethering from the displacement of the papillary muscle, and flail of multiple leaflets, cannot be corrected by annuloplasty alone.
1) Although several other techniques have been proposed, correction of TR with complex lesions is still demanding, and tricuspid valve replacement (TVR) could be required.
2) However, previous studies suggested that TVR results are insufficient compared with TV repair.
3)4)5)6)
Edge-to-edge repair (E2E), first introduced for mitral valve repair by Fucci et al.,
7) has been proposed to correct complex TV pathologies and showed favorable results.
8)9) E2E has several advantages for complex TV repair. First, E2E can be performed easily and quickly, even on a beating heart, without the aortic cross-clamp. Accordingly, it is helpful to correct the residual TR (
Supplementary Video 1). Second, E2E involves only the valve leaflets without distorting the right ventricle (RV) geometry, which is convenient for performing additional procedures. Third, E2E might be performed as an isolated procedure without annuloplasty when the patient has a small annulus prone to postoperative tricuspid stenosis (TS). In addition, E2E can be applied to various complex TV pathologies.
10)
Nevertheless, the comparative results of E2E repair and TVR have not been well investigated. Our study aimed to evaluate the long-term outcomes of E2E compared with TVR.
METHODS
Ethical statement
The study protocol was approved by the Bucheon Sejong Hospital Institutional Review Board (IRB), which waived the requirement for patient consent (IRB No. 2124; approval date, August 17, 2021).
Study population
We retrospectively reviewed 459 patients with severe TR who underwent E2E (n=264) or TVR (n=195) from 2001 to 2020. Among them, 229 patients were excluded due to the following conditions; less than severe TR, age <18 years, congenital heart disease except for atrial septal defects (including congenital TR, Ebstein anomaly, ventricular septal defect, tetralogy of Fallot, pulmonary atresia, congenitally corrected transposition of the great arteries, single ventricle), infective endocarditis, TS, previous TV repair, and previous TVR. The remaining 230 patients were categorized into E2E (n=139) and TVR (n=91) groups (
Supplementary Figure 1). Their mean age was 60.2±12.4 years, 169 patients (73.5%) were female, 177 patients (77.0%) had preoperative atrial fibrillation, and 60 patients (26.1%) had a history of previous cardiac surgery. Other baseline characteristics are described in
Table 1.
Table 1
Baseline characteristics
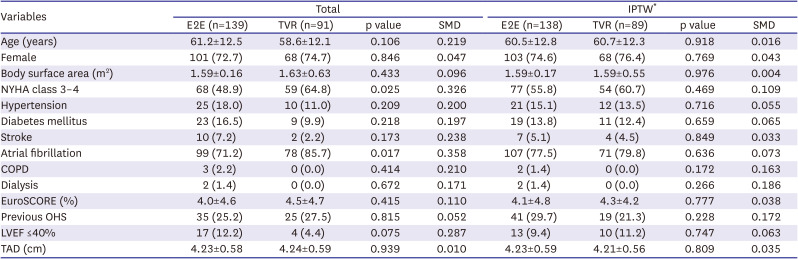
|
Variables |
Total |
IPTW*
|
|
E2E (n=139) |
TVR (n=91) |
p value |
SMD |
E2E (n=138) |
TVR (n=89) |
p value |
SMD |
|
Age (years) |
61.2±12.5 |
58.6±12.1 |
0.106 |
0.219 |
60.5±12.8 |
60.7±12.3 |
0.918 |
0.016 |
|
Female |
101 (72.7) |
68 (74.7) |
0.846 |
0.047 |
103 (74.6) |
68 (76.4) |
0.769 |
0.043 |
|
Body surface area (m2) |
1.59±0.16 |
1.63±0.63 |
0.433 |
0.096 |
1.59±0.17 |
1.59±0.55 |
0.976 |
0.004 |
|
NYHA class 3–4 |
68 (48.9) |
59 (64.8) |
0.025 |
0.326 |
77 (55.8) |
54 (60.7) |
0.469 |
0.109 |
|
Hypertension |
25 (18.0) |
10 (11.0) |
0.209 |
0.200 |
21 (15.1) |
12 (13.5) |
0.716 |
0.055 |
|
Diabetes mellitus |
23 (16.5) |
9 (9.9) |
0.218 |
0.197 |
19 (13.8) |
11 (12.4) |
0.659 |
0.065 |
|
Stroke |
10 (7.2) |
2 (2.2) |
0.173 |
0.238 |
7 (5.1) |
4 (4.5) |
0.849 |
0.033 |
|
Atrial fibrillation |
99 (71.2) |
78 (85.7) |
0.017 |
0.358 |
107 (77.5) |
71 (79.8) |
0.636 |
0.073 |
|
COPD |
3 (2.2) |
0 (0.0) |
0.414 |
0.210 |
2 (1.4) |
0 (0.0) |
0.172 |
0.163 |
|
Dialysis |
2 (1.4) |
0 (0.0) |
0.672 |
0.171 |
2 (1.4) |
0 (0.0) |
0.266 |
0.186 |
|
EuroSCORE (%) |
4.0±4.6 |
4.5±4.7 |
0.415 |
0.110 |
4.1±4.8 |
4.3±4.2 |
0.777 |
0.038 |
|
Previous OHS |
35 (25.2) |
25 (27.5) |
0.815 |
0.052 |
41 (29.7) |
19 (21.3) |
0.228 |
0.172 |
|
LVEF ≤40% |
17 (12.2) |
4 (4.4) |
0.075 |
0.287 |
13 (9.4) |
10 (11.2) |
0.747 |
0.063 |
|
TAD (cm) |
4.23±0.58 |
4.24±0.59 |
0.939 |
0.010 |
4.23±0.59 |
4.21±0.56 |
0.809 |
0.035 |

Operative techniques
The decision to perform E2E or TVR was based on the quality of the TV leaflets and the surgeon’s opinion on the clinical situation. If leaflet sclerosis is identified or the leaflet is too fragile, which results in a cut-through of E2E stitches, TVR was considered.
The detailed strategies for tricuspid E2E have been described previously.
10) For E2E, a stitch (5-0 polypropylene suture with or without pledgets) was placed to approximate leaflets’ free edges at the regurgitation site. Double-orifice repair
11) was performed in 78 patients. The clover technique
8) was performed in 61 patients. Concomitant TAP was performed in 121 patients (ring annuloplasty in 78 [64.5%]; suture annuloplasty in 43 [35.5%]). For suture annuloplasty, De Vega method was used in 42 patients (97.7%), and Kay’s procedure was used in 1 patient (2.3%). Suture annuloplasty was performed mainly in the earlier period of our valve surgery.
In the TVR group, a bioprosthesis was used in 59 (64.8%), and a mechanical prosthesis was used in 32 (35.2%) patients.
After weaning from cardiopulmonary bypass, transesophageal echocardiography was performed to reevaluate the valve function. The concomitant procedures and other operative data are presented in
Table 2.
Table 2
Operative data
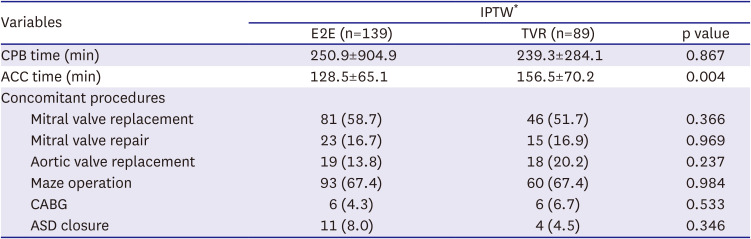
|
Variables |
IPTW*
|
|
E2E (n=139) |
TVR (n=89) |
p value |
|
CPB time (min) |
250.9±904.9 |
239.3±284.1 |
0.867 |
|
ACC time (min) |
128.5±65.1 |
156.5±70.2 |
0.004 |
|
Concomitant procedures |
|
|
|
|
Mitral valve replacement |
81 (58.7) |
46 (51.7) |
0.366 |
|
Mitral valve repair |
23 (16.7) |
15 (16.9) |
0.969 |
|
Aortic valve replacement |
19 (13.8) |
18 (20.2) |
0.237 |
|
Maze operation |
93 (67.4) |
60 (67.4) |
0.984 |
|
CABG |
6 (4.3) |
6 (6.7) |
0.533 |
|
ASD closure |
11 (8.0) |
4 (4.5) |
0.346 |

Follow-up
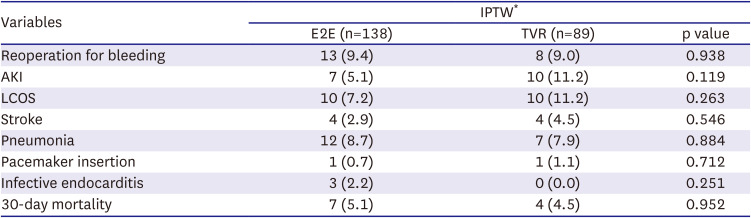
The primary endpoint of the study was all-cause mortality. The secondary endpoints were the presence of severe TR, significant TS, defined as trans-tricuspid pressure gradient (TTPG) ≥5 mmHg, TV reoperations, and TV-related events.
Early mortality was defined as death during hospitalization or within 30 days after the operation, while late mortality defined as death occurred after 30 days or after discharge. TV-related events included death, major bleeding, thromboembolism, valve thrombosis, structural or nonstructural prosthesis dysfunction, endocarditis, reoperation, and permanent pacemaker insertion.
Follow-up survival data were available for all patients, which were obtained from the institutional database (n=169, 73.5%) and the national registry (n=61, 26.5%). The mean follow-up duration was 106.2±68.8 months; 104.0±69.3 months in the E2E group vs. 109.5±68.2 months in the TVR group (p=0.556).
Echocardiographic assessment
According to the guidelines, the degree of TR was assessed using qualitative and semiquantitative methods with transthoracic Doppler echocardiography. The degree of TR was evaluated using the vena contracta width and the ratio of the maximal jet area to the corresponding right atrial area averaged on the parasternal and apical views. The severity of TR was graded on a scale from 0 to 3 (0, no or minimal; 1, mild; 2, moderate; and 3, severe).
12) The tricuspid annular diameter (TAD) was measured in the transthoracic apical 4-chamber view in late diastole at maximal TV opening.
13) The modified Bernoulli equation was used to calculate the TTPG from the maximal TR velocity measured by continuous-wave Doppler.
14)
Statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous variables are presented as means and standard deviations. Intergroup differences were assessed using the t-test (or the Mann–Whitney test when the normality assumption was in doubt) and the χ
2 test (or Fisher’s exact test when the expected cell frequency was <5). An inverse probability of treatment weighting (IPTW)-adjusted analysis was performed to balance the distribution of baseline risk factors between E2E and TVR groups. The propensity score (PS) was obtained by multiple logistic regression based on preoperative baseline characteristics. They included age, female sex, hypertension, diabetes mellitus, stroke, atrial fibrillation, chronic lung disease, New York Heart Association (NYHA) class 3–4, left ventricular ejection fraction <40%, EuroScore II, TAD, and concomitant operative procedures. Weights for patients with E2E were the inverse of the PS, and those for patients without E2E were the inverse of 1-PS. Stabilized weights were used to reduce variability in the IPTW model.
15) The density plots before and after IPTW were included in
Supplementary Figure 2. We also analyzed PS matching as an added robust analysis result. With this method, a total of 83 patients who underwent E2E were matched 1:1 with patients who underwent TVR using the PSs using nearest-neighbor matching without replacement and a matching tolerance (caliper) of 0.25 (
Supplementary Table 1). Survival curves were generated using the Kaplan-Meier method, and the survival rates were compared between the 2 groups and subgroups using the log-rank test. The Cox proportional hazards model analysis was employed to estimate the treatment effect of the 2 groups on long-term clinical outcomes. The hazard ratios (HRs) of late clinical outcomes between the 2 groups were compared based on original unmatched data, IPTW models, and matched data. Statistical significance was set at p<0.05. For modeling, the missing values of EuroScore II (16.9%) and TAD (16.9%) were imputed to the median of the non-missing values. All statistical analyses were performed using the R3.6.3 software (R Foundation for Statistical Computing, Vienna, Austria).
DISCUSSION
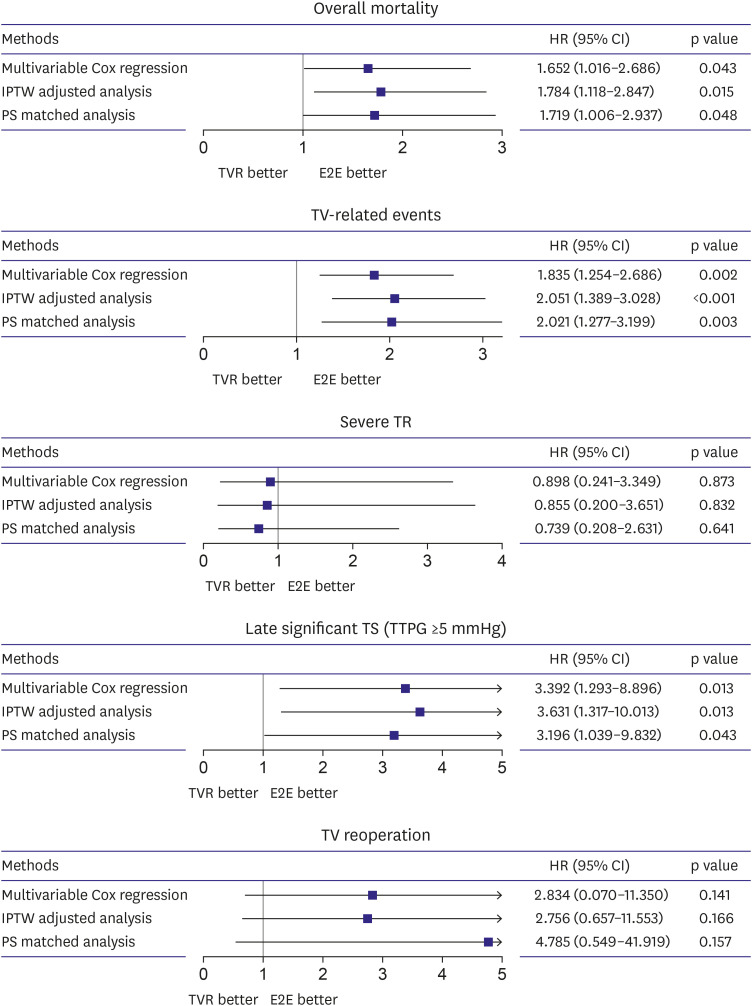
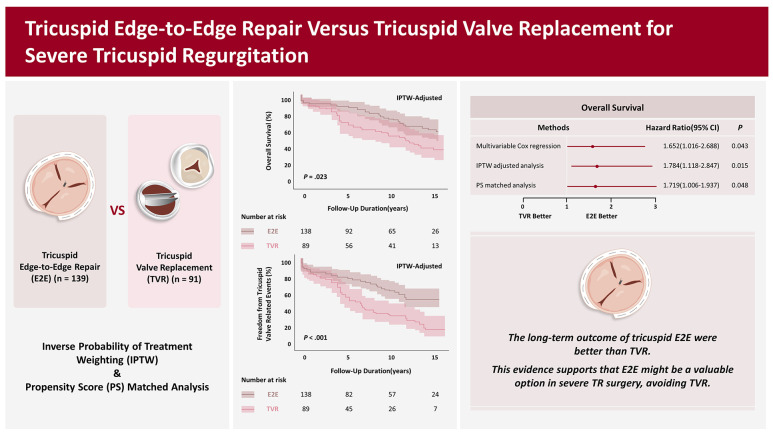
The main finding of the present study is that E2E showed better outcomes in overall mortality, TV-related events, and late significant TS. These results were consistent with various statistical methods.
Current guidelines recommend TV surgery in patients with severe TR undergoing left-sided valve surgery and in severe TR patients with either symptomatic right-sided heart failure or progressive RV dilation or systolic dysfunction.
16)17) The guidelines mentioned that TV repair is preferable to TVR, but specific indications for the type of TV surgery are unclear.
Several investigators also have suggested that TV repair is better than TVR. A recent meta-analysis by Choi et al.
5) showed that TVR had a higher risk for all-cause mortality (HR, 1.59; 95% confidence interval [CI], 1.26–2.00). Wong et al.
3) analyzed the outcomes of 2,644 patients who had undergone TV surgery (2,311 concomitant surgeries and 333 isolated TV surgery). In that study, TV repair demonstrated lower risks of all-cause mortality (HR, 0.76; 95% CI, 0.59–0.99), composite outcome (HR, 0.63; 95% CI, 0.46–0.86), and readmission (HR, 0.72; 95% CI, 0.60–0.86).
3) Marquis-Gravel et al.
18) investigated 926 patients who had received TV surgery (792 repairs and 134 replacements), and PS-adjusted analysis showed that TV repair had a lower risk for late mortality (HR, 0.52; p=0.02).
The most common type of TR is functional (secondary) TR with annular dilatation. Therefore, the current primary strategy for TR repair is TAP with an annuloplasty ring, which showed excellent long-term outcomes.
19)20) Meanwhile, TAP alone is insufficient for durable TV repair in complex TR etiologies such as the flail of multiple TV leaflets and severe leaflet tethering due to papillary muscle displacement. Several techniques have been proposed for these situations, such as leaflet augmentation, artificial chordae implantation, leaflet resection, chordal transposition, and papillary muscle reimplantation. Despite the proposed techniques, TV repair for complex TV pathology is still demanding and less reproducible, frequently leading to TVR.
21)
In contrast to other techniques which are technically challenging, E2E can be performed easily and quickly. The saline test is more reliable because E2E makes a fixed coaptation plane; the adequacy of leaflet coaptation can be analyzed more easily. An additional E2E stitch is considered if residual prolapse, leaflet retraction, or tethering is detected in the saline test after E2E. This stepwise approach is thought to be effective in preventing postoperative TS.
10) In addition, it is necessary to inspect the adequate valve opening area using a sucker tip or Hegar dilators. Nevertheless, when leaflet sclerosis is identified, or the leaflets are too fragile, which results in cut through with E2E, TVR needs to be considered.
Fucci et al.
7) first introduced the E2E technique for mitral regurgitation. Several groups have applied E2E for complex TV repair, which showed favorable outcomes. For instance, Lapenna et al. described 66 E2E cases for complex severe TR; the early mortality rate was 6%, and the 5-year survival rate was 91±4.1%. During the follow-up period (mean 3.5±1.6 years), 88.7% of the patients presented no or mild TR.
22) Our previous study demonstrated long-term outcomes of 237 patients with tricuspid E2E. Freedom from all-cause mortality was 80.6% at ten years, and freedom from moderate or severe was TR 84.9% at ten years.
10) However, previous studies have investigated little on comparative outcomes of E2E and TVR.
To our knowledge, this is the first study to compare the outcomes of E2E and TVR. The present study demonstrated superior long-term outcomes of E2E over TVR. Risks for overall mortality (1.652< HR <1.784), TV-related events (1.835< HR <2.051), and late significant TS (3.196< HR <3.631) were significantly higher in the TVR group. However, our study covers 20 years, and we acknowledge that our results may not solely be attributed to surgical techniques. Other factors, such as improved postoperative care or medications, could have contributed to the observed outcomes. To address this concern, we conducted an additional analysis using IPTW, including the surgery period. This analysis yielded results consistent with our primary analysis, as shown in
Supplementary Table 2 and
Supplementary Figure 5. These results suggest that E2E is a viable approach to prevent TVR in complex TR surgery.
In subgroup analysis according to the TV prosthesis types, the E2E group showed better outcomes than the bioprosthetic TVR group in overall survival, late significant TS, TV reoperation, and TV-related events. In contrast, no significant differences were identified between E2E and mechanical TVR groups. Nevertheless, the number of subgroups was small, so its statistical power may be limited. There has been vigorous debate on which type of valve (mechanical prosthesis vs. bioprosthesis) is suitable for TVR.
23)24)25) Valve types, institutional policy for medications, and anticoagulation strategy may influence the results. On this issue, large-scale randomized controlled trials are warranted.
Our study has several limitations. First, it was a retrospective, non-randomized study in a single institution. In order to address the issue, we utilized IPTW and PS matching. However, it is essential to note that the severity of right heart dysfunction/failure and the type of TR (secondary functional TR vs. isolated TR) can have a significant impact. Nevertheless, due to the retrospective nature of our study, we could not obtain data on the etiology of TR and RV dysfunction (e.g., tricuspid annular plane systolic excursion, RV fractional area change, and end-organ injury) for all patients. Therefore, selection bias or unknown confounding factors may have influenced our findings. Second, the selection of surgical strategy was mainly based on the surgeon’s preference, and it was impossible to define specific indications for TV repair or TVR for the study’s retrospective nature. Individual surgeon’s experience, anatomical factors of the TV, patient’s surgical risk, or concomitant procedures may have influenced the decision. Third, our study included heterogeneous patients and multiple valve pathologies. Future randomized studies would be necessary to confirm and validate our findings.
In this study, E2E for severe TR presented more favorable clinical outcomes than TVR. However, further research with a large randomized cohort is needed to confirm if E2E can be a viable alternative to TVR.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download