Abstract
Since its introduction, laparoscopic surgery has been often preferred over open surgery in obstetrics and gynecology due to its advantages, such as less bleeding, lower incidence of adhesions, reduced postoperative pain, short hospital stay, and quick return to daily life. However, in the case of complex surgeries, laparoscopy presented some limitations. Nonetheless, since the 1980s, medical robots have been introduced to overcome the technical limitations of laparoscopy and start a new age for minimally invasive surgery. In this review, we explore the indications and advantages and disadvantages of robotic surgery in the field of gynecology, and try to assess the recent trend of robotic surgery.
Recently the use of robotic technology has been expanding in surgical fields that require a high degree of precision, such as minimally invasive, brain, spine, and artificial joint surgeries. The da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA), which was approved by the United States. Food and Drug Administration (FDA) for the first time in the world as a robotic surgical system in 2000, ushered in the era of robotic surgery, popularizing it and promoting the development of relevant technologies. It was approved for use in gynecological surgery in 2005. Since its approval and introduction in the Republic of Korea (hereafter, Korea) in 2005, the da Vinci robotic surgical system has been used to perform surgeries in many fields, resulting in new treatment procedures and the accumulation of track records. Unlike the ZEUS system (Computer Motion, Santa Barbara, CA, USA), wherein the surgeon performs the surgery, such as laparoscopic surgery, while looking at a monitor, the da Vinci system allows the surgeon to perform the surgery under the real-time guidance of a stereoscopic image, and it allows free motion with 7 degrees of freedom rather than simply holding the laparoscopic instruments. However, it has some disadvantages in that the surgeon has a limited sense of touch and needs to constantly check the visual images, and the risk of hernia may increase because the trocar diameter is 8 mm larger than the diameter of the trocar used in conventional laparoscopic surgery [1]. Robotic-assisted surgeries are currently being performed in the gynecological field. This review aims to highlight the status of robotic-assisted surgery, indications for robotic-assisted surgery, and the differences between robotic-assisted surgery techniques and existing conventional surgical techniques.
The da Vinci surgical system has evolved from the first generation da Vinci S to the fourth generation da Vinci X, da Vinci Xi, and da Vinci single-port (SP). As of December 31, 2021, 4,139 da Vinci systems have been installed in the United States, 1,199 in Europe, 1,050 in Asia, and 342 in other countries. In total, it is estimated that about 1,594,000 surgeries have been completed using this system. According to a market research report, the global surgical robot market was expected to exceed KRW 20 trillion in 2021 [2].
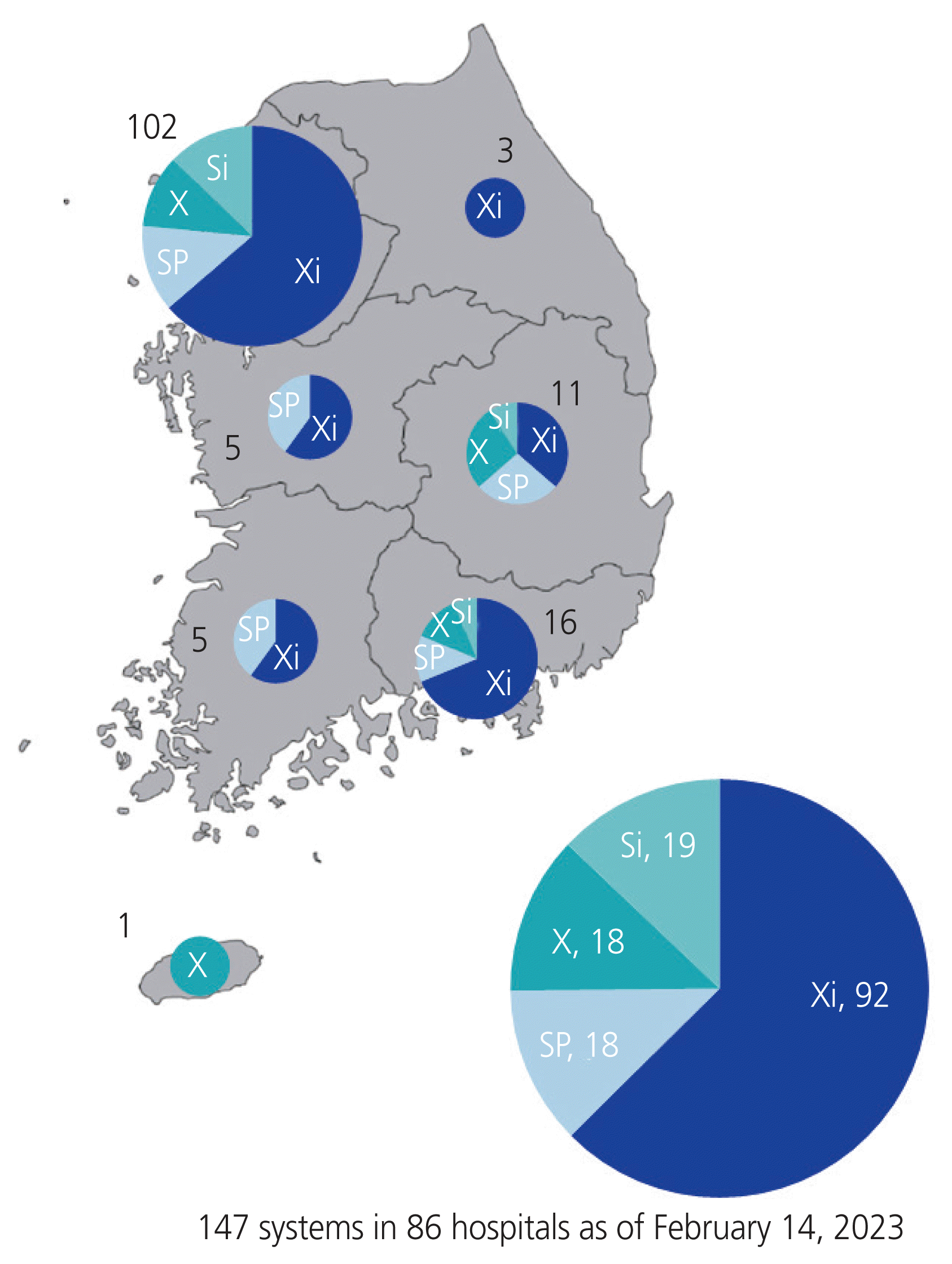
As of February 2023, a total of 147 da Vinci robot systems have been provided to 86 hospitals in Korea; fourth-generation models account for 63% of all installations, and 69% are concentrated in the Seoul metropolitan area (Fig. 1). Since the introduction of the robotic system in 2005 to 2022 in Korea, based on the number of surgeries by department, urology recorded the largest number of surgeries with 95,500 cases (37%), followed by obstetrics and gynecology with 64,200 cases (25%), the general surgery department with 46,400 cases (18%), otolaryngology with 43,000 cases (17%), thoracic surgery with 5,800 cases (2%), and other surgeries with 1,600 cases (1%).
Data were collected from four representative hospitals where robotic surgery is currently performed. Data from patients who underwent gynecological robotic surgeries (n=6,792) from 2009 to 2022 were collected and analyzed retrospectively. The patients who were operated on by a gynecologic oncologist or general gynecologist using the da Vinci® S, Si, X, Xi, or SP were included. The diagnosis was based on the diagnosis codes of the Health Insurance Review and Assessment Service. The year of introduction and the number of surgeries performed in each hospital are listed in Table 1.
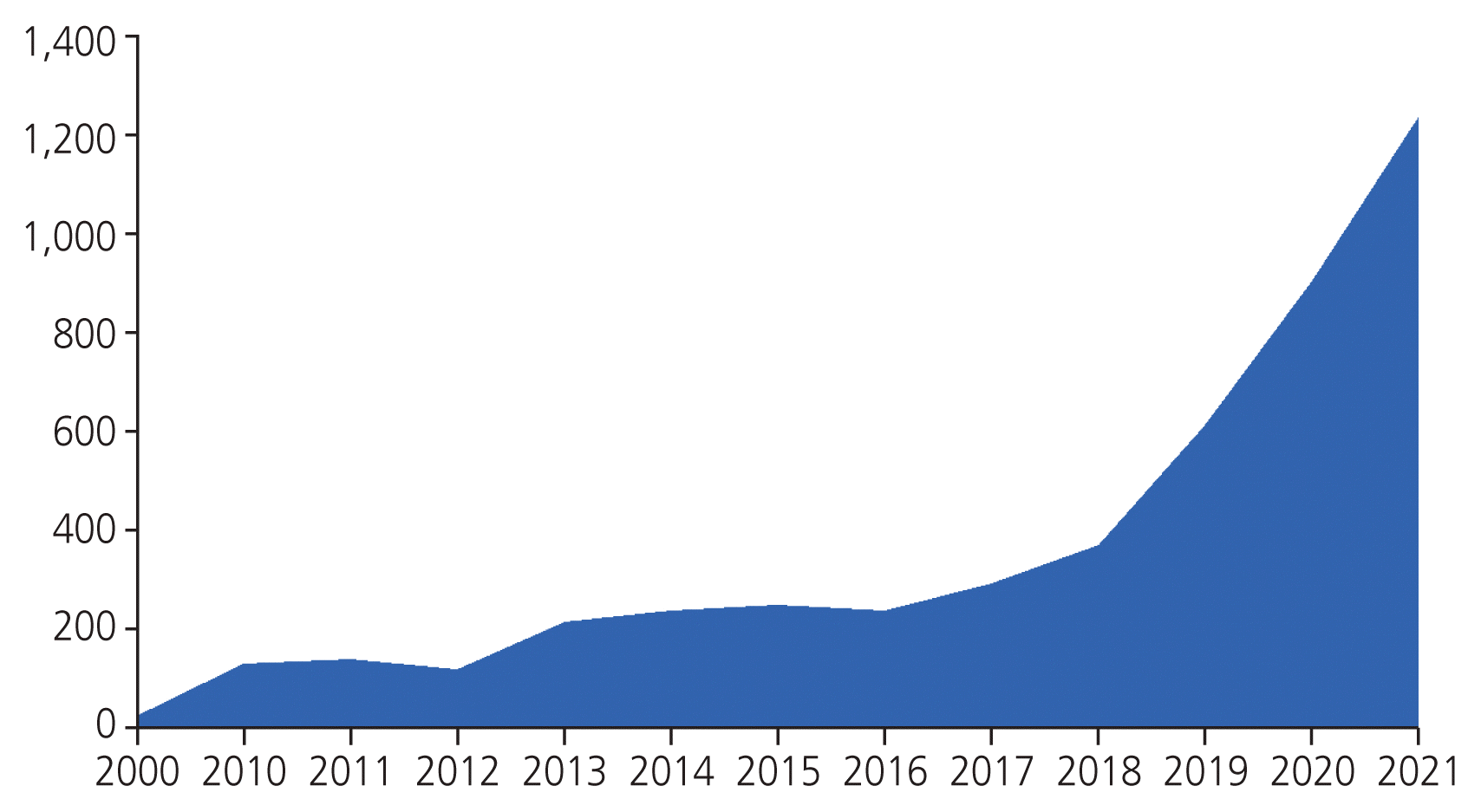
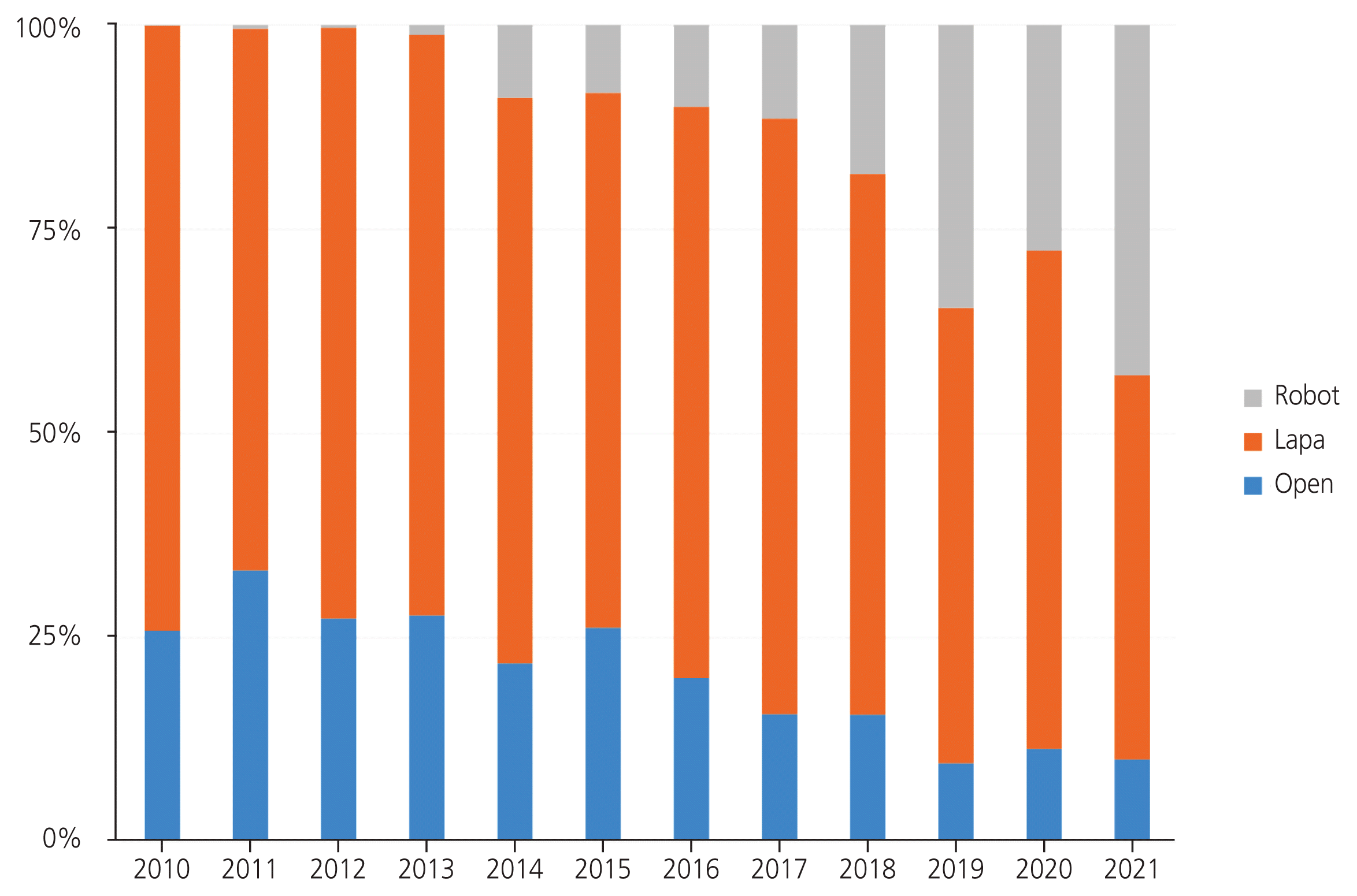
As of 2021, the number of robotic surgeries in obstetrics and gynecology grew rapidly by 782% (1,235 cases) compared to the surgeries from the previous 10 years ago, and since the introduction of da Vinci Xi in 2017 (the introduction of Xi in Korea was in 2014), it has increased further (Fig. 2).
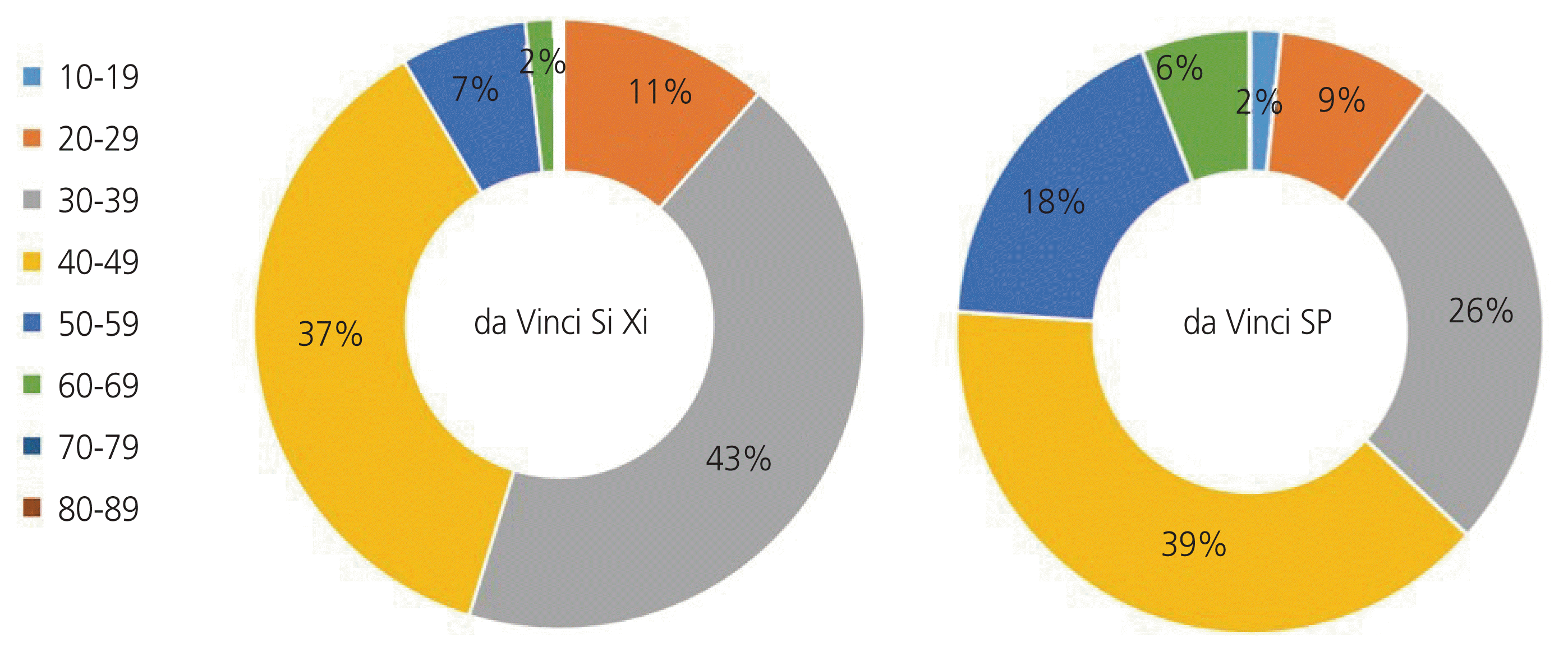
Based on the age distribution of patients who underwent robotic-assisted myomectomy, those in their 30s accounted for the highest proportion at 43%, followed by those in their 40s and 20s. In the case of da Vinci SP, which was introduced in 2021, the proportions of those in their 30s and 40s accounted for 26% and 39%, respectively. In the case of minimally invasive surgery (MIS) using robots, the surgical incision is smaller, and the recovery period is shorter; hence, it is highly preferred by young patients (Fig. 3).
Among the robotic obstetrics and gynecology surgeries, myomectomy (64%) accounted for the highest proportion, followed by hysterectomy (18%) and ovarian surgery (15%). In the case of myomectomy, which requires careful suturing, the introduction of robotic technology provides the same minimal invasiveness advantages as laparoscopic surgery and stability, as in open surgery; therefore, the popularity of robotic surgery is expected to increase (Fig. 4A).
Among the types of surgeries performed using the da Vinci SP system, which was introduced in 2021, ovarian surgery accounted for the highest proportion at 38%, followed by hysterectomy at 34%. The number of myomectomies accounted for 27%, which is lower than the proportion report for the previous version. The SP system does not include the tenaculum forceps that existed in the previous da Vinci model; hence, it is difficult to extract the fibroids, along with the difficulties associated with suction and irrigation during intraoperative bleeding. This system has a narrow range of free movements; hence, the proportion of myomectomies is low (Fig. 4B).
Hysterectomy is one of the most frequently performed surgical procedures in the United States, and common benign indications include symptomatic fibroids (51.4%), abnormal uterine bleeding (41.7%), endometriosis (30.0%), and uterine prolapse (18.2%) [3,4]. According to a 2015 Cochrane database systematic review, transvaginal hysterectomy has the advantage of a shorter time of return to normal activities than laparoscopic and abdominal hysterectomy. However, in the cases of adnexal pathology, severe endometriosis, adhesions, and enlarged uterus, vaginal access may be difficult [5]. Robotic surgery, which is the most advanced form of minimally invasive surgery, is showing a rapid increase in recent years, and its proportion is also increasing in hysterectomy [6]. According to a multicenter study in Korea in 2021, robotic-assisted hysterectomy accounted for 43% of all hysterectomies, showing a significant increase from 11% reported 5 years ago with a 4.6-fold increase in the total number of surgeries (Fig. 5).
A recent meta-analysis found that robotic and laparoscopic hysterectomies for benign disease showed no differences in the estimated blood loss, bleeding-related complications (hematomas, blood transfusions), length of hospital stay, postoperative pain levels, and recovery time before returning to daily life, and the total operating time (from incision to suture) [7]. Although the usefulness of robotic hysterectomy for benign diseases is still controversial in several randomized control trials, the postoperative quality of life (measured on a linear scale from 0 to 100) has been reported to be significantly higher in the robotic group [8]. In addition, a cohort study that evaluated the feasibility and safety of robot-assisted laparoscopic hysterectomy for patients with various body mass indexes showed that the complication or conversion rates did not increase significantly, even with the extended operating time, for obese patients. Furthermore, a study that compared robotic and abdominal hysterectomies for enlarged uterus weighing more than 1,000 g showed that the former had a longer operation time, shorter hospital stay, and less bleeding during surgery, suggesting the advantage of robotic-assisted surgery for a wide range of patients. In the case of difficult surgery due to an enlarged uterus or pelvic adhesions, it is believed that robots capable of anatomical approaches with excellent visualization can be advantageous, can be advantageous, but further long-term research is needed [9].
Uterine fibroids are the most common benign tumors in women of childbearing age, with various symptoms ranging from asymptomatic condition to hypermenorrhea, sensation of abdominal pressure including changes in urination and bowel habits, and infertility. It is known that fibroids are observed in 5–10% of infertile women, and their presence, regardless of the location, can lead to a significant increase in risk of miscarriage and infertility [10]. Despite the importance of fertility and quality of life, so far, there are limited treatments for fibroids, and there is no long-term drug treatment available. Myomectomy is the only treatment option for women interested in future fertility. Surgical treatment should be considered because intramural fibroids larger than 4 cm, including submucosal fibroids, can negatively impact fertility [11]. Laparoscopic myomectomy, or open myomectomy, can be performed to treat more than one fibroid, and robotic-assisted myomectomy has been introduced as a minimally invasive surgical treatment since the mid-2000s. A recent meta-analysis showed that robotic-assisted myomectomy showed better results regarding the number of complications, etimated blood loss, number of blood transfusions, and length of hospital stay but longer surgery duration than open myomectomy. In addition, the conversion rate to laparotomy was much lower than that of laparoscopic surgery. The incidence of complications was higher for laparoscopic and open surgeries than that of robotic-assisted surgery, mainly because of the improved three-dimensional (3D) vision system of the robotic surgical system, enabling ergonomic and precise suturing in less time using wrist instruments. In addition, robotic-assisted myomectomy effectively improved patients’ quality of life. However, further studies are needed to observe differences in long-term outcomes (e.g., postoperative pain, and fertility) [12]. Although there were no significant differences in the fertility rates between the patients who underwent laparoscopic and robotic-assisted surgeries, improved surgical procedures, reduced risk of complications, and other positive long-term outcomes can be expected as surgeons become more proficient in robotic-assisted surgery with time [13].
Robotic single-port myomectomy was introduced in October 2018 in Korea and has attracted attention since then. In one study that compared robotic single- and multi-port myomectomy, fewer fibroids were extracted with the former, and the sum of the maximum diameters of the removed fibroids was small. However, there was no significant difference in hospital stay and rate of complications between the two approaches, and the pain score on the first day after surgery was lower with the single-part approach. This allows for a wide variety of treatment options depending on the patient’s characteristics and the type of disease [14].
Endometriosis is a common benign disease with a prevalence of 6–10% among women of childbearing age that can causes various symptoms such as dysmenorrhea, chronic pelvic pain, dyspareunia, and infertility. Its symptoms may be accompanied by urinary and intestinal symptoms, depending on the location of the lesions [15]. Endometriosis can be classified according to the depth and location of infiltration, and if the depth is more than 5 mm, it is classified as deep infiltrative endometriosis (DIE) [16]. Many studies have agreed that surgical removal of DIE improves pain and the quality of life. Previously, pathologic confirmation followed by laparoscopic surgery was considered the standard treatment for endometriosis. However, the European Society of Human Reproduction and Embryology guidelines published in February 2022 recommend laparoscopic surgery only if previous empirical treatments have failed [17]. A meta-analysis comparing laparoscopic surgery with robotic-assisted surgery showed no significant difference in the length of hospital stay, rate of intra- and postoperative complications, conversion rate, and blood loss volume between the two approaches. Robotic surgery had a longer average surgical time than laparoscopy [18,19]. However, the introduction of robotic surgery has provided new perspectives on DIE, as it allows correction with 3D imaging and access to complex anatomical structures, thus improving surgical performance without increasing surgical time, bleeding, and the risk of perioperative complications. It even has a low conversion rate to laparotomy and a short learning curve for surgeons. With these advantages, robotic surgery may be the best option for treating DIE [20].
Pelvic organ prolapse (POP) is a condition in which a woman’s pelvic organs, including the bladder, uterus, vagina, or rectum, have descended from their normal position in the pelvis. These symptoms manifest in 30% of women aged between 50 and 89 years, and about 11% of women under the age of 80 years require corrective procedures. POP often occurs as a process of aging in women with a history of natural birth or hysterectomy and in women with increased body mass index whose ligaments and muscles that support the pelvis are weakened. The lifetime risk of recurrence of POP after surgical treatment is 13–19% [21]. A Cochrane review comparing surgical techniques to treat POP found that abdominal sacrocolpopexy (ASC) is an effective treatment method for apical vaginal prolapse. It has longer surgical and recovery durations, and higher costs than transvaginal sacrospinous ligament fixation but lower rates of recurrence of vault prolapse and postoperative dyspareunia. Laparoscopic sacrocolpopexy causes relatively little postoperative pain and has a short recovery time, but the reduced degree of freedom and increased operation time due to laparoscopic access are the main limitations. Robotic-assisted surgical techniques can overcome the limitations of laparoscopy given their features, such as 3D visualization, increased degrees of freedom, and improved ergonomic environment. Several studies comparing laparoscopic and robotic-assisted surgery showed that laparoscopic surgery led to higher postoperative pain scores, higher analgesic demands, and longer surgery times [22]. In other studies, robotic and laparoscopic surgeries showed reduced blood loss compared to ASC. However, long-term data on the rates of success and revision owing to complications are needed [23].
There is no consensus on the optimal surgical treatment for recurrent POP. Some researchers prefer the vaginal approach to treat relapse of POP [21]; sacrocolpopexy can be a treatment option because the FDA prohibits using a transvaginal prosthetic mesh. In a single-center study, robotic-assisted sacrocolpopexy did not cause perioperative complications such as early bladder or bowel injury, indicating that it can help correct POP while minimizing the risk of complications from fibrosis or adhesion. The median operative time also showed no clear difference from the time for laparoscopic reconstruction [24,25]. Robotic sacrocolpopexy is a minimally invasive technique that is useful for POP patients. This shows excellent short-term reconstructive outcomes and provides a good surgery environment for surgeons. Therefore, it is expected to be a good treatment option for POP.
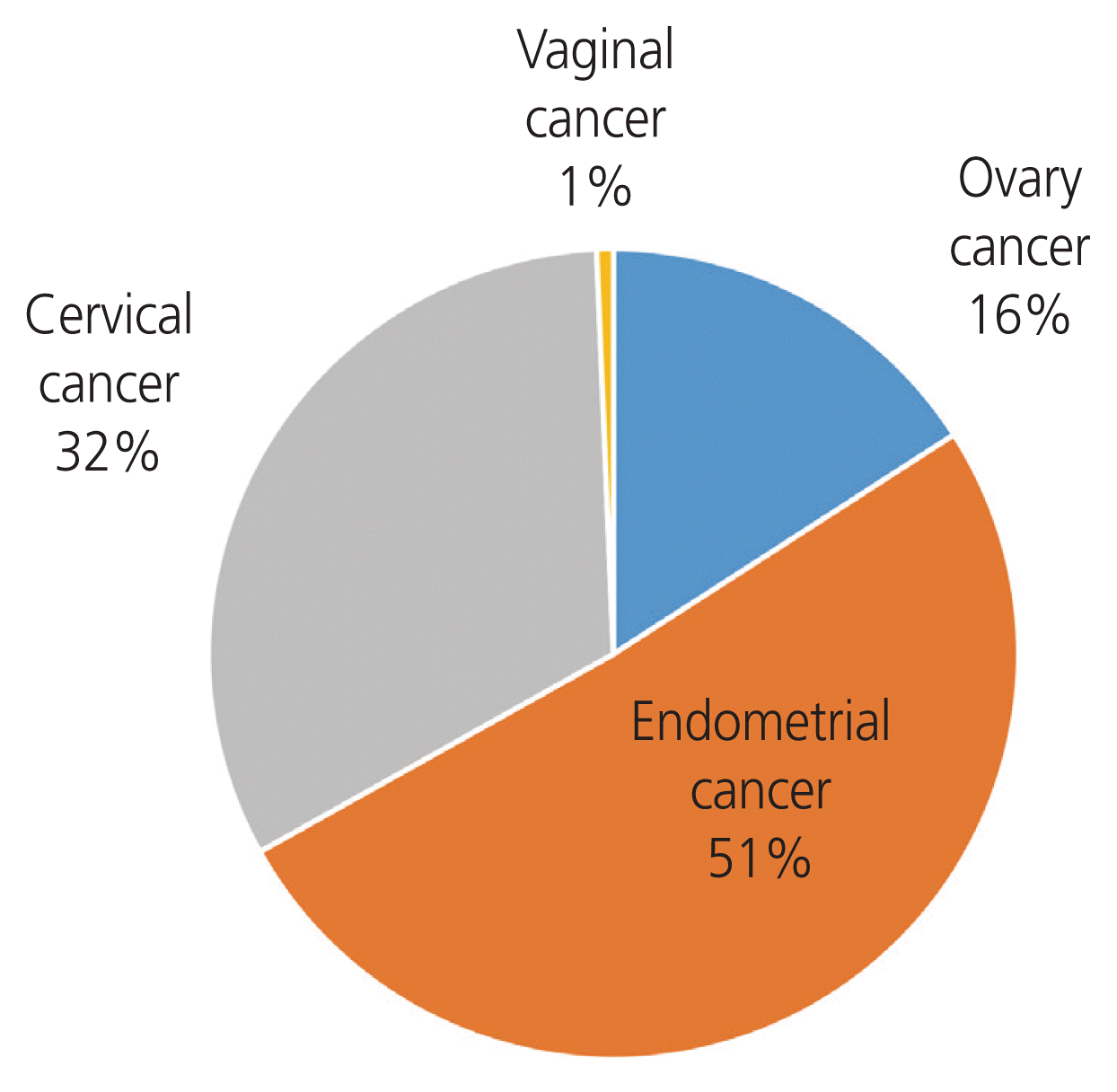
Robotic surgery is also widely used to treat gynecologic cancers given its high precision and stability. Among cancers treated with robotic surgery, endometrial cancer accounted for the highest proportion at 51%, followed by cervical and ovarian cancer (Fig. 6). In the case of cervical cancer, the results of the recent laparoscopic approach to carcinoma of the cervix (LACC) clinical trial of cervical cancer patients reported that MIS led to a shorter length of disease-free survival and survival than extensive open hysterectomy, and it was usually performed in the early stages of the disease. Recently, the proportion of cervical cancer is showing a decreasing trend [26].
For treating endometrial cancer, MIS is considered the first choice. Two large prospective randomized controlled trials, “laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology group LAP2”, based on gynecologic oncologists in the United States, and “laparoscopic approach to cancer of the endometrium”, based in Australia, New Zealand, Hong Kong, and Scotland, reported that compared to open surgery, laparoscopic surgery reduced the length of stay in the hospital, blood loss, and the number of antiemetics [27–39].
Some studies reported that robotic surgery takes longer, but it usually has a shorter operating time than laparoscopic surgery [40–42]. With experienced surgical teams, the difference in operating time between robotic and laparoscopic surgery is considered insignificant compared to the advantages of robotic surgery [41]. In Denmark, the MIS rate has increased after the introduction of robotic surgery. Robotic surgery systems are expensive in Korea, and the high cost makes it difficult to popularize them to the same level as in Denmark, but the reduction in complications can justify the installation of expensive robotic systems [43,44].
Recently, there has been a trend of avoiding full lymph node (LN) dissection. Although sentinel nodes, ultra-staging, etc., can be performed differently at each center, there is a trend of not performing or performing sentinel when there is no suspicion of LN metastasis to prevent lymphatic edema and patients’ quality of life worsening. For the removal of sentinel LNs, robotic surgery is usually preferred [45–47].
In the early stage of cervical cancer, patients can choose MIS for radical hysterectomy or radical trachelectomy. Trachelectomy is primarily performed on patients with cervical cancer who need fertility preservation. The fertility outcome may be an essential selection criterion for choosing between open surgery and MIS. Studies have shown that robotic surgery outcomes are better than open surgery outcomes, making it an option for patients. However, when choosing a surgical approach for cervical cancer surgery, the LACC trial results can make clinicians rethink the decision to select robotic surgery [26,48]. Many clinicians choose radical hysterectomy via laparotomy in light of the results of the LACC trial. As with other diseases, robots have become more common, and robot-assisted approach to cervical cancer trials are being conducted to create results that can compete with this trial. Research is also being conducted in China [49].
The debulking operation for ovarian cancer takes a long time because the lesion is usually located in a large area in the abdominal cavity and requires staging. Nevertheless, it usually has a poor prognosis, and most patients undergo adjuvant chemotherapy after surgery. In recent years, neoadjuvant chemotherapy has been followed by cytoreductive interval surgery based on the results of the INTERNATIONAL MISSION study [50]. After neoadjuvant chemotherapy, MIS is also performed if the response is excellent, depending on the cell type. From the surgical point of view, there are often cases, such as early uterine cancer, in which there are no obvious lesions in the abdominal cavity other than the primary lesion. Therefore, robotic surgery can also be applied to ovarian cancer. Abitbol et al. [51] reported that interval robotic cytoreduction can be an option for selected patients. MIS is the universal choice for uterine cancer, but in addition to the LACC trial of cervical cancer, the treatment of ovarian cancer is cytoreductive surgery via laparotomy [51]. The MIRRORS study (Minimally Invasive Robotic Surgery, Role in Optimal Debulking Ovarian Cancer, Recovery & Survival) recruited patients with stage IIIC and IV ovarian cancer and presented the robotic-assisted interval cytoreductive surgery results at the European Society of Gynaecological Oncology 2022 [52]. We expect the results of the prospective study. Iavazzo et al. [53] mentioned that robotic surgery can be used for treating ovarian cancer. However, research on patient selection is needed.
The field of robotic surgery has made significant progress in the last decade, and the application of robotic surgery in gynecological surgery is becoming increasingly common. As robotic technology develops, there will be further modifications to this system. In addition, the advantages of robotic surgery over conventional surgical techniques are clear, but the limitations related to cost, extended operation time, and anesthesia time remain. However, ongoing technological advancements can overcome these limitations. Robotic-assisted MIS is expected to be the leading modality in the field of gynecological surgery in the future. Prospective directions for research and development in robotic surgery include the use of smaller robotic equipment, assisted docking, the introduction of single-incision surgery, the ability to perform remote surgery using robotic systems, and the reduction of the setup and surgical time. Further prospective research is needed to provide additional information on the long-term results and the cost-effectiveness of robotic-assisted surgical techniques.
Notes
References
1. Watrowski R, Kostov S, Alkatout I. Complications in laparoscopic and robotic-assisted surgery: definitions, classifications, incidence and risk factors - an up-to-date review. Wideochir Inne Tech Maloinwazyjne. 2021; 16:501–25.

2. Intuitive Corp. Intuitive annual report Intuitive Surgical, Inc [Internet]. Sunnyvale: Intuitive Corp;c2021. [cited 2023 Apr 18]. Available from: https://isrg.intuitive.com/static-files/704322bf-cb0d-4ed1-954c-8eb46a070f70
.
3. Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008; 198:34.e1–7.

4. Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013; 309:689–98.

5. Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015; 2015:CD003677.

6. Committee opinion no. 701 summary: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017; 129:1149–50.
7. Albright BB, Witte T, Tofte AN, Chou J, Black JD, Desai VB, et al. Robotic versus laparoscopic hysterectomy for benign disease: a systematic review and meta-analysis of randomized trials. J Minim Invasive Gynecol. 2016; 23:18–27.

8. Sarlos D, Kots L, Stevanovic N, von Felten S, Schär G. Robotic compared with conventional laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012; 120:604–11.
9. Silasi DA, Gallo T, Silasi M, Menderes G, Azodi M. Robotic versus abdominal hysterectomy for very large uteri. JSLS. 2013; 17:400–6.

10. Sohn GS, Cho S, Kim YM, Cho CH, Kim MR, Lee SR. Current medical treatment of uterine fibroids. Obstet Gynecol Sci. 2018; 61:192–201.

11. Zepiridis LI, Grimbizis GF, Tarlatzis BC. Infertility and uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016; 34:66–73.

12. Wang T, Tang H, Xie Z, Deng S. Robotic-assisted vs. laparoscopic and abdominal myomectomy for treatment of uterine fibroids: a meta-analysis. Minim Invasive Ther Allied Technol. 2018; 27:249–64.

13. Cooper AR, Powell MA, Jimenez PT, Johnson MD, Rabinov A, Graseck AS. Discussion: ‘comparison of robotic and laparoscopic myomectomy’ by Bedient et al. Am J Obstet Gynecol. 2009; 201:e1–4.

14. Ahn SH, Park JH, Kim HR, Cho S, Lee M, Seo SK, et al. Robotic single-site versus multi-port myom4ectomy: a case-control study. BMC Surg. 2021; 21:264.
15. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010; 362:2389–98.
16. de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017; 69:587–96.

17. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: endometriosis. Hum Reprod Open. 2022; 2022:hoac009.
18. Magrina JF, Espada M, Kho RM, Cetta R, Chang YH, Magtibay PM. Surgical excision of advanced endometriosis: perioperative outcomes and impacting factors. J Minim Invasive Gynecol. 2015; 22:944–50.

19. Restaino S, Mereu L, Finelli A, Spina MR, Marini G, Catena U, et al. Robotic surgery vs laparoscopic surgery in patients with diagnosis of endometriosis: a systematic review and meta-analysis. J Robot Surg. 2020; 14:687–94.

20. Cela V, Obino ME, Sergiampietri C, Simi G, Papini F, Pinelli S, et al. The role of robotics in the management of endometriosis. Minerva Ginecol. 2017; 69:504–16.

21. Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010; 116:1096–100.

22. Seror J, Yates DR, Seringe E, Vaessen C, Bitker MO, Chartier-Kastler E, et al. Prospective comparison of short-term functional outcomes obtained after pure laparoscopic and robot-assisted laparoscopic sacrocolpopexy. World J Urol. 2012; 30:393–8.

23. Ploumidis A, Spinoit AF, De Naeyer G, Schatteman P, Gan M, Ficarra V, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: surgical technique and outcomes at a single high-volume institution. Eur Urol. 2014; 65:138–45.

24. Dabreteau T, Delangle R, Azaïs H, Phé V, Moawad G, Uzan C, et al. Robot-assisted sacrocolpopexy for recurrent pelvic organ prolapse: insights for a challenging surgical setting. J Gynecol Obstet Hum Reprod. 2022; 51:102380.

25. Maher CM, Feiner B, Baessler K, Glazener CM. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011; 22:1445–57.

26. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018; 379:1895–904.

27. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009; 27:5331–6.

28. Kornblith AB, Huang HQ, Walker JL, Spirtos NM, Rotmensch J, Cella D. Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J Clin Oncol. 2009; 27:5337–42.

29. Feuer GA, Lakhi N, Barker J, Salmieri S, Burrell M. Perioperative and clinical outcomes in the management of epithelial ovarian cancer using a robotic or abdominal approach. Gynecol Oncol. 2013; 131:520–4.

30. Kumar S, Al-Wahab Z, Sarangi S, Woelk J, Morris R, Munkarah A, et al. Risk of postoperative venous thromboembolism after minimally invasive surgery for endometrial and cervical cancer is low: a multi-institutional study. Gynecol Oncol. 2013; 130:207–12.

31. Mahdi H, Aljebori Q, Lockart D, Moulton L. Risk of venous thromboembolism after laparoscopic surgery for gynecologic malignancy. J Minim Invasive Gynecol. 2016; 23:1057–62.

32. Gildea C, Nordin A, Hirschowitz L, Poole J. Thirty-day postoperative mortality for endometrial carcinoma in England: a population-based study. BJOG. 2016; 123:1853–61.

33. Moss EL, Morgan G, Martin AP, Sarhanis P, Ind T. Surgical trends, outcomes and disparities in minimal invasive surgery for patients with endometrial cancer in England: a retrospective cohort study. BMJ Open. 2020; 10:e036222.

34. Barber EL, Gehrig PA, Clarke-Pearson DL. Venous thromboembolism in minimally invasive compared with open hysterectomy for endometrial cancer. Obstet Gynecol. 2016; 128:121–6.

35. Capozzi VA, Sozzi G, Gambino G, Cianciolo A, Riccò M, Monfardini L, et al. Laparoscopy versus laparotomy for surgical treatment of obese women with endometrial cancer: a cost-benefit comparative analysis. Mol Clin Oncol. 2019; 11:335–42.

36. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012; 30:695–700.

37. Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: a randomized clinical trial. JAMA. 2017; 317:1224–33.

38. Uwins C, Tailor A, Chatterjee J, Ellis P, Madhuri T, Michael A, et al. 254 Robotic corpus cancer surgery: ten year mortality data from the UK epicentre in guildford. Int J Gynecol Cancer. 2020; 30:A31–2.
39. Galaal K, Donkers H, Bryant A, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2018; 10:CD006655.

40. Wang J, Li X, Wu H, Zhang Y, Wang F. A meta-analysis of robotic surgery in endometrial cancer: comparison with laparoscopy and laparotomy. Dis Markers. 2020; 2020:2503753.

41. Mäenpää MM, Nieminen K, Tomás EI, Laurila M, Luukkaala TH, Mäenpää JU. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: a randomized controlled trial. Am J Obstet Gynecol. 2016; 215:588.e1–7.
42. Chung H, Jang TK, Nam SH, Kwon SH, Shin SJ, Cho CH. Robotic single-site staging operation for early-stage endometrial cancer: initial experience at a single institution. Obstet Gynecol Sci. 2019; 62:149–56.

43. Jørgensen SL, Mogensen O, Wu CS, Korsholm M, Lund K, Jensen PT. Survival after a nationwide introduction of robotic surgery in women with early-stage endometrial cancer: a population-based prospective cohort study. Eur J Cancer. 2019; 109:1–11.

44. Jørgensen SL, Mogensen O, Wu C, Lund K, Iachina M, Korsholm M, et al. Nationwide introduction of minimally invasive robotic surgery for early-stage endometrial cancer and its association with severe complications. JAMA Surg. 2019; 154:530–8.

45. Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008; 100:1707–16.

46. Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2017; 10:CD007585.

47. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009; 373:125–36.

48. Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018; 379:1905–14.

49. Chao X, Li L, Wu M, Ma S, Tan X, Zhong S, et al. Efficacy of different surgical approaches in the clinical and survival outcomes of patients with early-stage cervical cancer: protocol of a phase III multicentre randomised controlled trial in China. BMJ Open. 2019; 9:e029055.

50. Fagotti A, Gueli Alletti S, Corrado G, Cola E, Vizza E, Vieira M, et al. The INTERNATIONAL MISSION study: minimally invasive surgery in ovarian neoplasms after neoadjuvant chemotherapy. Int J Gynecol Cancer. 2019; 29:5–9.

51. Abitbol J, Gotlieb W, Zeng Z, Ramanakumar A, Kessous R, Kogan L, et al. Incorporating robotic surgery into the management of ovarian cancer after neoadjuvant chemotherapy. Int J Gynecol Cancer. 2019; 29:1341–7.

52. Uwins C, Tailor A, Read J, Crawshaw J, Chatterjee J, Ellis P, et al. 2022-RA-1649-ESGO mirrors study: a prospective cohort study assessing the feasibility of robotic interval cytoreductive surgery for advanced-stage ovarian cancer. Int J Gynecol Cancer. 2022; 32:A357–8.
Fig. 4
Analysis of robotic surgery types in obstetrics and gynecology in multiport (A) and SP system (B).
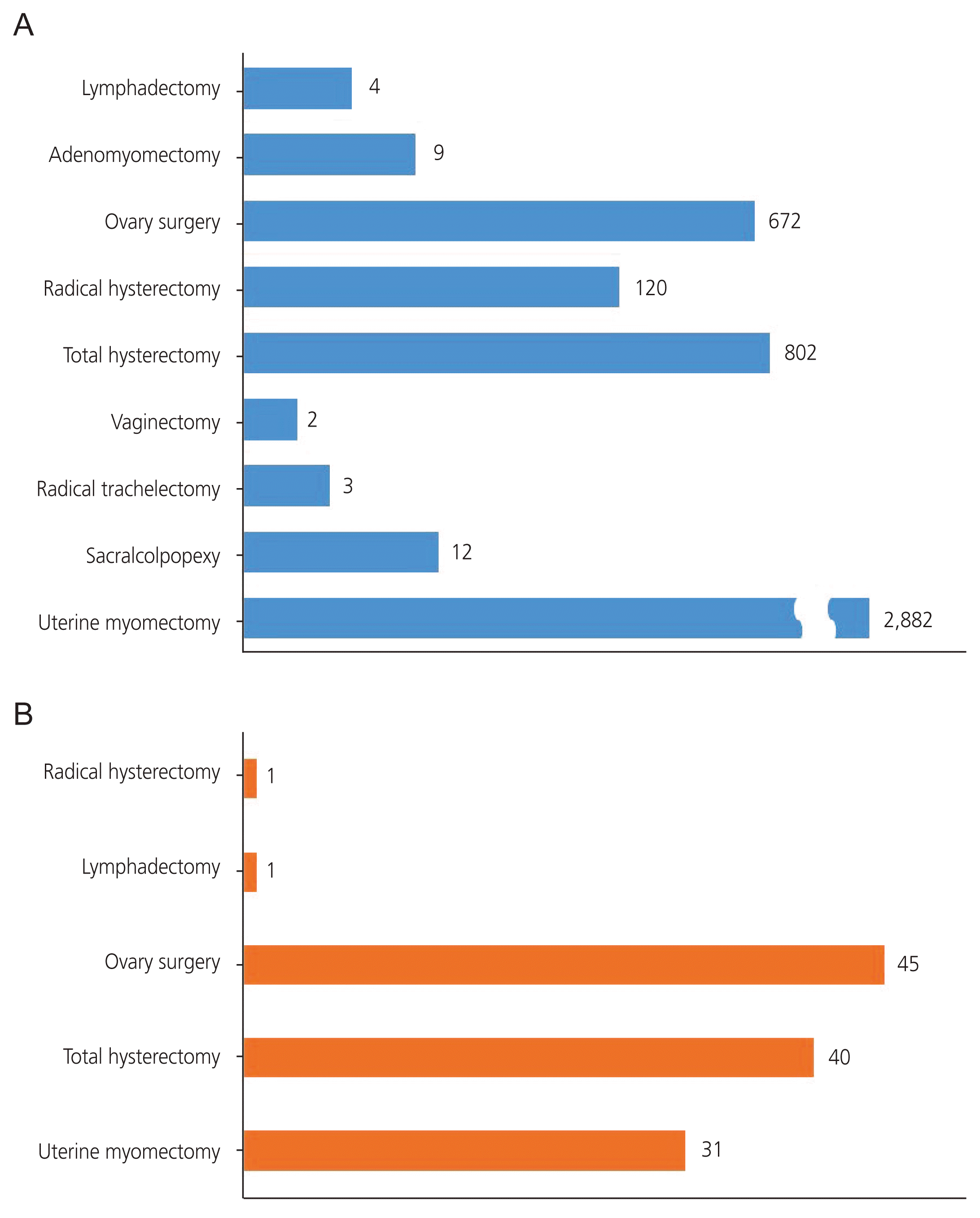
Table 1
Features of each hospital’s robotic system




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download