Introduction
Method
Results
Table 1
| Study | No. of patients | Tumor type/stage | Op method | LND protocol | Tracer | Ultrastaging |
|---|---|---|---|---|---|---|
| Negishi et al. (2004) [10] | 11 | Endometrial cancer (n=10), fallopian tube cancer (n=1) | LT | CH40 (activated charcoal solution) | - | |
| Nyberg et al. (2011) [19] | 16 | Uterine cancer (normal ovaries) | LT | Tc-99m+blue dye | - | |
| Kleppe et al. (2014) [14] | 21 | Ovarian mass (suspicious malignancy) | LT |
Benign or borderline cases, SLN tracing only Malignant cases, SLN followed by pelvic+paraaortic LND |
Tc-99m+blue dye | - |
| Buda et al. (2017) [16] | 10 | Early ovarian cancer (n=7), cervical cancer (n=3) | LS | SLN followed by pelvic+paraaortic LND | ICG | - |
| Angelucci et al. (2016) [25] | 5 | Early ovarian cancer/I–II | LS | SLN followed by pelvic+paraaortic LND | ICG | - |
| Hassanzadeh et al. (2016) [15] | 35 | Ovarian mass (suspicious malignancy) | LT |
Benign or borderline cases, SLN tracing only Malignant cases, SLN followed by pelvic+paraaortic LND |
Tc-99m±blue dye (used in 4 patients) | - |
|
Lago et al. (2019) [34] Pilot study of SENTOV triala) |
10 | Ovarian cancer (confirmed by previous surgery or frozen section) |
LT (n=7) LS (n=3) |
SLN followed by pelvic+paraaortic LND | Tc-99m+ICG | - |
|
Uccella et al. (2019) [9] Preliminary results of SELLY trialb) |
31 | Early ovarian cancer/I–II |
LT (n=1) LS (n=26) RB (n=4) |
SLN followed by pelvic+paraaortic LND | ICG | + |
|
Lago et al. (2020) [20] SENTOV trial phase IIc) |
20 | Ovarian cancer (confirmed by previous surgery or frozen section) |
LT (n=11) LS (n=9) |
SLN followed by pelvic+paraaortic LND | Tc-99m+ICG | - |
|
Laven et al. (2021) [19] SONAR-2 trial phase Id) |
11 | Ovarian cancer (confirmed by previous surgery [n=3] or frozen section [n=8]) | LT | SLN followed by pelvic+paraaortic LND | Tc-99m+blue dye | - |
Table 2
| Study | Tracer | Tracer dosage | Injection site | Injection time | Interval from injection to detection | SLN location | Detection rates | Sensitivity | NPV |
|---|---|---|---|---|---|---|---|---|---|
| Negishi et al. (2004) [10] | CH40 (activated charcoal solution) | 0.05–0.2 mL | Ovarian cortex | Before adnexectomy | 10 minutes |
Paraaortic (91.0%) Common iliac (26.0%) External iliac (9.0%) |
100.0% | - | - |
| Nyberg et al. (2011) [19] | Tc-99m+blue dye | 19 MBq (0.9 mL)+2.0 mL | Ovarian hilum | 15 minutes before adnexectomy | 138 minutes | Paraaortic only (100.0%) | 94.0% | - | - |
| Kleppe et al. (2014) [14] | Tc-99m+blue dye | 20 MBq+0.2 mL-0.5 mL | Ovarian ligament & IP ligament | 15 minutes before adnexectomy | 50 minutes |
Paraaortic only (67.0%) Pelvic only (9.0%) Pelvic/paraaortic (24.0%) |
100.0% | - | - |
| Buda et al. (2017) [16] | ICG | 0.5 mL-1.0 mL (1.25 mg/mL) | Ovarian ligament & IP ligament | Before adnexectomy | - |
Paraaortic only (70.0%) Pelvic only (10.0%) Pelvic/paraaortic (20.0%) |
90.0% | No positive node | 100.0% |
| Angelucci et al. (2016) [25] | ICG | 0.5 mL-1.0 mL (1.25 mg/mL) | Ovarian hilum | Before adnexectomy | 2 minutes |
Paraaortic only (40.0%) Pelvic only (60.0%) |
100.0% | No positive node | 100.0% |
| Hassanzadeh et al. (2016) [15] | Tc-99m±blue dye (used in 4 patients) | 37 MBq (0.2 mL)+0.2 cc |
Ovary cortex (n=10) Ovarian ligament & IP ligament (n=25) |
10 minutes before adnexectomy | - |
Paraaortic only (87.5%) Pelvic only (8.3%) Pelvic/paraaortic (8.3%) |
40.0% 84.0% |
NA 100.0% |
- |
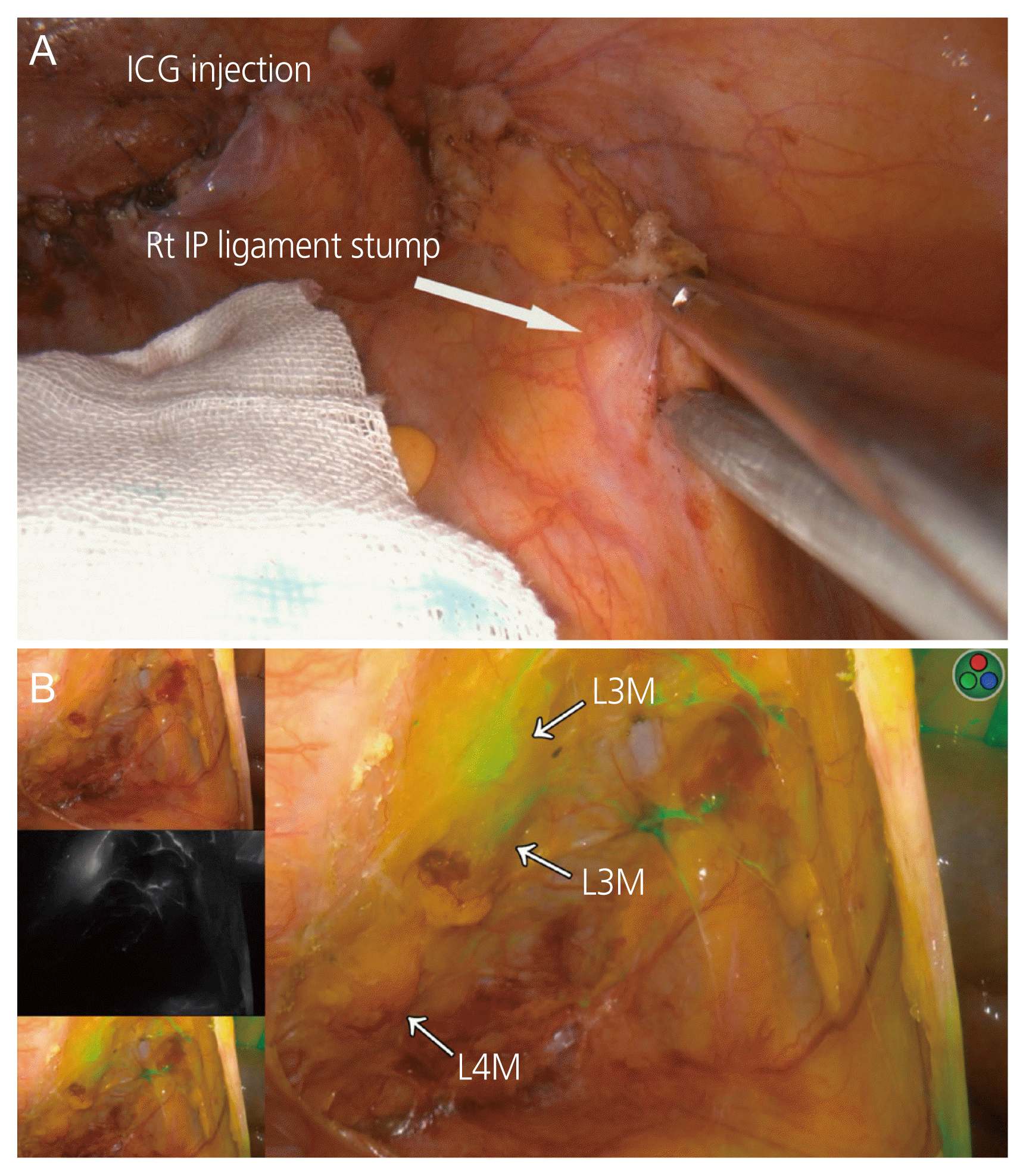
| Lago et al. (2019) [34] | Tc-99m+ICG | 37 MBq (0.2 mL)+0.5 mL (1.25 mg/mL) | IP ligament stump±ovarian ligament stumpa) | After adnexectomy | 15 minutesb) |
Paraaortic (70.0%) Pelvic (87.5%) |
Both: 100.0% (Tc-99m alone: 100.0%), (ICG alone: 90.0%) | - | - |
| Uccella et al. (2019) [9] | ICG | 2.0 mL (1.25 mg/mL) | IP ligament stump±ovarian ligament stumpa) | After adnexectomyc) | 5–20 minutes |
Paraaortic only (62.0%) Pelvic only (19.0%) Pelvic/paraaortic (19.0%) |
67.7% (immediate, 88.9%), (delayed, 41.7%) | 100.0% (specificity 100.0%) | 100.0% (PPD 100.0%) |
| Lago et al. (2020) [20] | Tc-99m+ICG | 37 MBq (0.2 mL)+0.5 mL (1.25 mg/mL) | IP ligament stump±ovarian ligament stumpa) | After adnexectomy | 15 minutesb) |
Paraaortic (100.0%) Pelvic (93.3%) Pelvic/paraaortic (95.0%) |
Both: 100.0% (Tc-99m alone: 100.0%), (ICG alone: 95.0%) | No positive node | - |
| Laven et al. (2021) [17] | Tc-99m+blue dye | 20 MBq (0.15 mL)+2.0 mL | IP ligament stump+ovarian ligament stump | After adnexectomyc) | 15 minutes |
Paraaortic only (67.0%) Pelvic only (0.0%) Pelvic/paraaortic (33.0%) |
27.3% | No positive node | - |
1. Injection tracer
2. Blue dye
3. Tc-99m
4. ICG
5. Injection site
6. Ovarian cortex
7. IP ligament (or ligament stump) and ovarian ligament (or ligament stump)
8. Injection time: before or after tumor resection
9. SLN location
10. Ultrastaging and detection accuracy of SLN
11. Complications associated with SLN biopsy
12. Perspectives for future research
Table 4
| Study (trial registration number) | Estimated enrollment | Tumor type/stage | LND protocol | Injection site | Injection time | Tracer | Tracer dosage | Ultrastaging |
|---|---|---|---|---|---|---|---|---|
| SONAR-2 (NCT02540551) [19]a) | 11 (actual enrollment) | Ovarian cancer (confirmed by previous surgery [n=3] or frozen section [n=8]) | SLN followed by pelvic+paraaortic LND | IP ligament stump+ovarian ligament stump | After adnexectomy (immediate or delayed) | Tc-99m+blue dye | 20 MBq (0.15 mL)+2.0 mL | - |
| SENTOV (NCT03452982) [20,29,34]b) | 20 | Ovarian cancer (confirmed by previous surgery or frozen section) | SLN followed by pelvic+paraaortic LND | IP ligament stump±ovarian ligament stump (if no previous hysterectomy) | After adnexectomy | Tc-99m+ICG | 37 MBq (0.2 mL)+0.5 mL (1.25 mg/mL) |
-,Afterwards, ultrastaging was performed in pilot study and clinical trial patients Regular intervals of 200 μm, with a limit of 6 levels If absence of metastasis, IHC (anticytokeratin AE1:AE3) |
| SELLY (NCT03563781) [9] | 176 | Early epithelial ovarian cancer/I–II | SLN followed by pelvic+paraaortic LND | IP ligament stump±ovarian ligament stump (if no previous hysterectomy) | After adnexectomy (immediate or delayed) | ICG | 2.0 mL (1.25 mg/mL) | +,The negative ones are ultrastaged following a protocol based on multiple H&E sections combined with IHC (anticytokeratin AE1:AE3) |
| MELISA (NCT05184140) [35]c) | 62 | Ovarian mass (suspicious of malignancy), ovarian cancer (re-staging) | Malignant cases, SLN followed by pelvic+paraaortic LND | IP ligament stump±ovarian ligament stump | Tc-99m injection (preoperative)+ ICG injection (after adnexectomy when frozen section proven malignancy) | Tc-99m+ICG | 37–74 MBq (0.4 mL/injection)+0.3 mL −1.0 mL (2.5 mg/mL) |
+,4 μm thick, regular intervals of 150 μm, performing 4 levels of each paraffin block If absence of metastasis, IHC (anticytokeratin AE1:AE3) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download