Abstract
Background and Objectives
This study focused on predicting voice outcomes following hemithyroidectomy using skin electrode intraoperative neuromonitoring (IONM).
Subjects and Method
The study involved 82 patients who underwent hemithyroidectomy. During the surgery, the researchers recorded skin IONM values for the vagus nerve and recurrent laryngeal nerve. Voice quality evaluations were conducted before the surgery and at various intervals after the surgery (one week, one month, three months, six months, and one year) using the multidimensional voice program (MDVP), voice handicap index (VHI), and the grade, roughness, breathiness, asthenia, strain (GRBAS) scale. The study aimed to correlate perioperative skin IONM values with subjective and objective voice outcomes.
Results
The VHI scores increased up to three months post-surgery, with statistically significant changes observed at one week post-surgery. The GRBAS scale scores also increased significantly at one month post-surgery. Similar trends were observed in the MDVP data, including a decrease in the maximum pitch between one week and three months post-surgery. Additionally, the study found that lower delta-V values (difference between the preoperative and postoperative signal amplitude of vagus nerve stimulation) were associated with higher F0 values at one week and three months post-surgery, while jitter and noise-to-harmonic ratio were higher in the group with higher delta-V values at three months post-surgery.
Conclusion
Greater differences between the preoperative and postoperative signal amplitude of vagus nerve stimulation during surgery were linked to worse postoperative voice outcomes. Voice parameters worsened for up to 3 months after hemithyroidectomy but showed signs of recovery afterwards. These findings offer insights into predicting and managing voice outcomes after hemithyroidectomy.
The prevalence of thyroid cancer has significantly increased over the past few decades [1]. Increased accessibility to ultrasound and fine-needle aspiration cytology has led to early detection of thyroid cancer, including papillary thyroid microcarcinoma. Thus, hemithyroidectomy is being increasingly performed [2]. Owing to the development of surgical techniques, most patients with thyroid cancer have a good prognosis. Therefore, patients expect positive changes to their quality of life, especially their voice, after surgery.
Post-thyroidectomy voice disorder (PTVD) occurs in 25%-84% of thyroidectomies [3]. According to Lee, et al., the most common cause of voice change is damage to the recurrent laryngeal nerve (RLN) and external branch of the superior laryngeal nerve (EBSLN) [4]. RLN damage can cause hoarse voice, voice fatigue, and breathing voices, whereas EBSLN damage makes it difficult to produce high-frequency tones [5,6]. Other causes of voice impairment include cricothyroid muscle injury, strap muscle injury, endotracheal intubation-related trauma, vascular congestion, and local hematoma [7-10]. PTVD after thyroid surgery is well known; however, limited data are available on PTVD after hemithyroidectomy, which has been increasingly performed nowadays.
No international consensus has been reached on the diagnostic criteria for PTVD, and a few criteria have been used in previous studies (Fig. 1). When voice handicap index (VHI) score >12 is used to define PTVD, the incidence of PTVD increases to 24% 1 week after surgery followed by falling to less than 8% after 3 months [11,12]. According to grade, roughness, breathiness, asthenia, strain (GRBAS) score >0, it is highest at 6% 1 month after surgery, followed by a decreasing trend [12]. Pitch maximum (Pmax) <80% of its preoperative value is used as a diagnostic criterion and is taken as a standard of Pmax. It is highest at 11% 1 month after surgery followed by a decreasing trend [3].
In recent years, several efforts have been made to prevent voice impairment after thyroid surgery. In South Korea, an electromyographic (EMG) endotracheal tube may be used as an intraoperative neuromonitoring (IONM) device if the National Health Insurance standards are met, which include the following: 1) recurrent thyroid cancer in the central neck region, 2) unilateral vocal cord palsy before thyroid surgery, 3) thyroid cancer with definitive central neck lymph node metastasis, 4) thyroid cancer with definitive or suspicious extra-thyroid extension, and 5) high-risk thyroid surgery and parathyroid surgery, such as surgeries performed for Graves’ disease or significant thyroid enlargement. IONM can help identify the anatomical disposition of the nerves near the thyroid, which helps prevent damage to the RLN and EBSLN [5]. However, owing to the high cost ($250 in South Korea), considerable rate of false ‘Loss of signal’ (10%-15%) as well as limited indications, most surgeons perform thyroid surgery without IONM.
Therefore, other types of IONM are needed. Recent studies have shown that electrodes attached to the skin can complement the EMG endotracheal tube [13], and skin electrode IONM in thyroid surgery is increasingly used.
Voice changes may occur even after hemithyroidectomy; however, no methods are available to predict it. Therefore, in this study, we tried to determine whether PTVD could be predicted using the parameters obtained by skin IONM and subjective and objective voice test results of patients. Moreover, the incidence and clinical progress of PTVD after hemithyroidectomy were investigated.
Eighty-two patients who underwent hemithyroidectomy using skin electrode IONM between June 2019 and December 2020 were retrospectively included in this study. The exclusion criteria for enrollment were: 1) preoperative vocal cord palsy or paresis; 2) vocal cord disease (such as polyps, nodules, papillomas, and Reinke’s edema); 3) laryngopharyngeal reflux; 4) complaints of hoarseness; 5) preoperative evidence of lateral nodal disease; and 6) insufficient medical records. The surgery was performed by a single senior surgeon (Kwang Yoon Jung). The Institutional Review Board (IRB) of the Korea University Medical Center approved this study (IRB No. 2023AN0157).
Two pre-gelled surface electrodes (DSE3125; Medtronic Xomed Inc, Jacksonville, FL, USA), sized 1.5×2.0×2.5 cm were attached to the skin at the level of the upper lateral margin of the thyroid cartilage bilaterally. The amplitudes of the four signals were measured using a neuromonitoring device (Computerized Speech Lab [CSL], Model 4500; KayPentax Inc, Lincoln Park, NJ, USA) with a ball-tip stimulator (Probe). Before thyroid lobe removal, the V1 parameter was acquired from the vagus nerve in the carotid sheath with a stimulation power of 5.0 mA while the R1 parameter was obtained from the RLN with a power of 2.0 mA. When the thyroid lobe was removed and the RLN was exposed, the R2 parameter from the exposed RLN was obtained with a power of 1.0 mA, and the V2 parameter from the vagus nerve was obtained with a power of 5.0 mA (Table 1).
The vocal cords of all patients were examined before and after thyroidectomy. The subjects underwent repeated functional voice quality evaluation before surgery and at 1 week, 1 month, 3 months, 6 months, and 1 year postoperatively. The patients received voice therapy during the same postoperative period. Two voice specialists who were unaware of the study performed the voice quality evaluations.
Acoustic analysis (measuring fundamental frequency [F0], jitter, shimmer, and noise-to-harmonic ratio [NHR]) was performed by the multidimensional voice program (MDVP) software application from the Computerized Speech Lab system (model 4500; KayPentax Inc, Lincoln Park, NJ, USA). Aerodynamic analysis (measuring mean flow rate, maximum phonation time, and subglottic pressure) and voice range profile analysis (measuring voice pitch range and intensity range) were also performed.
The subjective voice was evaluated using the GRBAS scale, which consists of five parameters: overall grade of hoarseness (G), roughness (R), breathiness (B), asthenia (A), and strain (S). A 4-point scale was used for each parameter (0=normal, 1=slight, 2=moderate, 3=severe). The VHI questionnaire, which consists of 30 questions divided by content into 3 subscales covering functional, physical, and emotional parameters was used as well. The scale was scored from 0 (never) to 4 (always) for each question, with a minimum score of 0 and a maximum total score of 120.
All statistical analyses were performed using the SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). Statistical analysis was performed using the Wilcoxon signed-rank test to compare voice outcomes by period, and paired t-test and Mann-Whitney U test were used to compare IONM parameters and voice analysis outcomes. For all the tests, the statistical significance was set at a probability of <0.05 (p<0.05).
A total of 82 patients were enrolled in the study. The mean age was 50.9 (±14.3) years. Among 82 patients, 18 were male and 64 were female. The mean body mass index was 24.89 (±3.57) kg/m2. Seventy-two patients were diagnosed with papillary thyroid carcinoma, while the others were diagnosed with nodular hyperplasia, sclerosing thyroiditis, Hurthle cell adenoma, or follicular adenoma. The average size of the tumor was 0.80 (±0.55) cm. An extrathyroidal extension was observed in six patients during thyroid surgery (Table 2).
The amplitude of the skin IONM parameters was as follows: the average value of V1 was 237.57 (±81.69) μV, while the median was 227 (range, 106-419) μV. The average values of V2, R1, and R2 were 216.32 (±88.16) μV, 176.63 (±70.07) μV, and 192.96 (±83.16) μV, respectively, while the median values were 202 (range, 74-512) μV, 157 (range, 48-379) μV, 167.5 (range, 91-462) μV, respectively. Delta V (ΔV), defined as the difference between V1 and V2 (V1-V2) had an average value of 21.26 (±61.89) μV with a median value of 19.5 (range, -275-154) μV (Table 3).
Changes in voice parameters after hemithyroidectomy are denoted on various scales (Fig. 2 and Table 4). In the early postoperative period, some voice parameters worsened. Compared to the preoperative value (2.500), the VHI score increased until postoperative 3 months, though only the changes occurring in the postoperative 1 week (8.317, p<0.001) were statistically significant. The GRBAS scale score also showed an increase during postoperative 1 month (0.146, p=0.039) compared to the preoperative score (0.024).
MDVP showed a similar trend. The Pmax decreased during the period of postoperative 1 week, 1 month and 3 months (p<0.001, p<0.001, and p=0.005, respectively). During the late postoperative period, F0 (Hz) increased and jitter (%) decreased at postoperative 1 year (both p=0.001). Shimmer (%) decreased during the period between postoperative 6 months and 1 year (p=0.001 and p<0.001, respectively). The NHR and pitch minimal (Pmin) did not show any significant changes perioperatively.
To compare IONM parameter amplitudes and perioperative voice parameters, the patients were divided into two groups according to whether the patient’s ΔV was higher or lower than the median value (19.5 μV). When voice outcomes of the two groups are compared, no statistically significant differences were observed in VHI and GRBAS scores between the two groups (Fig. 3 and Table 5). However, when ΔV was lower than the median value, F0 was higher than its values at postoperative 1 week and 3 months (both p=0.04). Jitter and NHR had a higher figure in the group with ΔV higher than the median value at postoperative 3 months (p=0.02 and p=0.001, respectively). A subgroup analysis was performed based on the sex of the patients, and no significant differences were observed.
PTVD is known to have various causes, such as nerve damage, muscle strap and constraint, esophageal adhesion, and vocal cord damage during anesthesia, which can occur even in the absence of obvious nerve damage. The literature to date has included data on the occurrence of voice disorders after thyroidectomy, and limited data are available on voice disorders after lobectomy, which has been increasingly performed. A meta-analysis showed that IONM during thyroid surgery significantly reduces damage to the RLN [14]. In this study, we investigated the incidence and progression of voice disorders after lobectomy and evaluated whether the occurrence of PTVD could be predicted through IONM using skin electrodes.
When the skin IONM parameters were measured, the average value of R2 was higher than that of R1. Unlike R1, R2 is a value measured after RLN exposure; therefore, it showed a higher signal value even with a lower-intensity stimulus. Thus, we used the difference in V values that were measured at the same location and using the same-intensity stimulus.
Extensive data are available on IONM parameter values using endotracheal tubes, and standard values are being established; however, data on IONM parameter values using skin electrodes are limited. Prior research has demonstrated that the median amplitude of vagus nerve stimulation using the attached electrodes typically measures around 200 μV, which is less than that of the EMG tube [15]. Our current study yielded comparable results (Table 2), with the reduced intensity attributed to signal attenuation during the transmission of signals from the vocal cord muscles to the skin.
While previous research has examined the pre- and postoperative amplitude ratios of R1 to R2 and V1 to V2 [15], no prior studies have explored the connection between intraoperative nerve monitoring parameters and voice outcomes. In this study, we hypothesized that a larger difference in amplitude was associated with more severe nerve damage and worse voice change. Therefore, patients were divided into two groups using the median change in the V value as the cut-off value.
Contrary to expectations, no statistically significant difference was observed in the subjective parameters between the two groups. Until postoperative 1 month, when the subjective parameters showed deterioration (Fig. 2), only F0 significantly decreased in the group with larger ΔV. At postoperative 3 months, F0, jitter, and NHR worsened in the group with larger ΔV, and no significant difference was observed between the groups after that period. To evaluate the differences caused by sex when measuring F0, Pmax, and Pmin, we conducted a subgroup analysis in the male and female groups; however, no significant differences were observed. From these results, if the difference between V1 and V2 is large, we can predict that the voice analysis results might be worse than their counterparts within postoperative 3 months followed by recovery after postoperative 6 months.
Voice alterations following thyroid surgery are a well-acknowledged phenomenon, extending beyond instances of RLN injury [16]. A comparative investigation involving pre- and postoperative voice metrics, similar to our own study, revealed that post-operative voice impairment is most pronounced immediately following the surgical procedure. Subsequently, there is a noteworthy improvement at the three-month mark, although it remains inferior to pre-operative levels. However, it tends to fully return to pre-operative status within one year [17].
After thyroidectomy, adhesions form between the larynx and the adjacent strap muscles and subcutaneous fat, constricting laryngeal mobility and precipitating voice alterations [18]. Notably, this study ascertained a significant restoration of voice quality at the three-month juncture in comparison to the one-month post-operative assessment, a pattern that aligns with our present investigation.
Furthermore, it is noteworthy that voice impairment can manifest even in the absence of overt RLN damage, attributable to adhesions with the surrounding tissues of the larynx. Such impairments tend to exhibit discernible recovery trends approximately three months post-surgery. The extent of the difference in amplitude between pre- and postoperative vagus nerve stimulation values can impact voice quality. Nevertheless, even if the voice initially deteriorates, it often tends to recover over time.
A limitation of our study is that when using a skin electrode, the measured value may be different for each person depending on the location of attachment and skin thickness; therefore, our research results cannot be generalized. In this study, every operation was performed by a single senior surgeon. Therefore, the inter-tester errors were minimized.
In our study, only 82 patients were examined; therefore, we attempted to predict voice changes using median cut-off values. Further studies with a larger number of patients should be conducted to establish standardized values to predict voice outcomes using skin electrode IONM.
We used the V values in this study instead of the R values, which have a more direct effect on the voice owing to different experimental settings. In future studies, we might measure R values with the same power before and after hemithyroidectomy to obtain meaningful results in predicting voice changes using delta R values. A subsequent study may also consider conducting a comparison of both latency and amplitude in order to arrive at more inclusive findings. Furthermore, the investigation could extend to evaluating the parameters of the superior laryngeal nerve, which has the potential to influence high-pitched voice production, thereby contributing to a more comprehensive assessment of voice prognosis.
In conclusion, early diagnosis of thyroid cancer has become possible because of the early implementation of ultrasound and fine-needle aspiration cytology, and hemithyroidectomy is being increasingly performed. This study was conducted to predict the incidence and course of the voice disorders that occur after hemithyroidectomy.
Several studies have attempted to predict the incidence of PTVD after thyroidectomy using various scales. The incidence tended to increase until 1 month after surgery and was reported to be up to 24%. Subsequently, it showed a tendency to gradually decrease, and after 3 months, it was usually normalized. This study showed a trend consistent with previous studies and revealed that voice outcomes deteriorated until 3 months after surgery and then gradually improved.
We attempted to predict post-hemithyroidectomy voice changes using IONM parameters with skin electrodes. IONM using a skin electrode can be used during thyroid surgery in cases where the EMG endotracheal tube cannot be used because of limited indications. As shown in this study, it may be possible to predict the occurrence of PTVD in patients based on the ΔV value measured during surgery. A greater difference in the amplitude of the IONM parameter was associated with the likelihood of PTVD occurrence. Further studies are required to include more patient groups with more detailed subgroup analyses to reveal the association between various voice parameters and PTVD, such that the operators are able to predict PTVD more accurately and ultimately improve voice outcomes after thyroidectomy.
Notes
Author Contribution
Conceptualization: Minsu Kwon, Jae Gu Cho, Seung Kuk Baek, Kwang Yoon Jung. Data curation: Hyeon Geun Kim. Formal analysis: Hyeon Geun Kim. Investigation: Dabin Lee. Methodology: Dabin Lee, Hyeon Geun Kim, Minsu Kwon, Kwang Yoon Jung. Project administration: Kwang Yoon Jung. Resources: Dabin Lee, Kwang Yoon Jung. Supervision: Minsu Kwon, Jae Gu Cho, Kyoung Ho Oh, Seung Kuk Baek, Soon Young Kwon, Jeong Soo Woo, Kwang Yoon Jung. Visualization: Kwang Yoon Jung. Writing—original draft: Dabin Lee. Writing—review & editing: Dabin Lee.
REFERENCES
1. Lubitz CC, Sosa JA. The changing landscape of papillary thyroid cancer: Epidemiology, management, and the implications for patients. Cancer. 2016; 122(24):3754–9.

2. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000; 160(4):526–34.

3. Lee DY, Lee KJ, Hwang SM, Oh KH, Cho JG, Baek SK, et al. Analysis of temporal change in voice quality after thyroidectomy: Single-institution prospective study. J Voice. 2017; 31(2):195–201.

4. Chandrasekhar SS, Randolph GW, Seidman MD, Rosenfeld RM, Angelos P, Barkmeier-Kraemer J, et al. Clinical practice guideline: Improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg. 2013; 148(6 Suppl):S1–37.

5. Randolph GW, Dralle H, Abdullah H, Barczynski M, Bellantone R, Brauckhoff M, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: International standards guideline statement. Laryngoscope. 2011; 121(Suppl 1):S1–16.

6. Barczyński M, Randolph GW, Cernea CR, Dralle H, Dionigi G, Alesina PF, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope. 2013; 123(Suppl 4):S1–14.

7. Lang BH, Wong CK, Ma EP. A systematic review and meta-analysis on acoustic voice parameters after uncomplicated thyroidectomy. Laryngoscope. 2016; 126(2):528–37.

8. Teitelbaum BJ, Wenig BL. Superior laryngeal nerve injury from thyroid surgery. Head Neck. 1995; 17(1):36–40.

9. Soylu L, Ozbas S, Uslu HY, Kocak S. The evaluation of the causes of subjective voice disturbances after thyroid surgery. Am J Surg. 2007; 194(3):317–22.

10. Kark AE, Kissin MW, Auerbach R, Meikle M. Voice changes after thyroidectomy: Role of the external laryngeal nerve. Br Med J (Clin Res Ed). 1984; 289(6456):1412–5.

11. Lee YW, Kim GH. Discriminative and predictive ability for screening the Korean dysphonic patients using self-reported questionnaires. Clin Arch Commun Disord. 2020; 5(2):85–95.

12. Heikkinen M, Penttilä E, Qvarnström M, Mäkinen K, Löppönen H, Kärkkäinen JM. Perceptual assessment and acoustic voice analysis as screening tests for vocal fold paresis after thyroid or parathyroid surgery. World J Surg. 2021; 45(3):765–73.
13. Lee HS, Oh J, Kim SW, Jeong YW, Wu CW, Chiang FY, et al. Intraoperative neuromonitoring of recurrent laryngeal nerve during thyroidectomy with adhesive skin electrodes. World J Surg. 2020; 44(1):148–54.

14. Zheng S, Xu Z, Wei Y, Zeng M, He J. Effect of intraoperative neuromonitoring on recurrent laryngeal nerve palsy rates after thyroid surgery--a meta-analysis. J Formos Med Assoc. 2013; 112(8):463–72.

15. Seo SG, Lee HS, Jo KH, Kim SW, Lee KD. Efficacy of intraoperative neuromonitoring during thyroidectomy with transcutaneous adhesive skin electrodes. Korean J Otorhinolaryngol-Head Neck Surg. 2021; 64(6):416–21.

16. Lombardi CP, Raffaelli M, D’Alatri L, Marchese MR, Rigante M, Paludetti G, et al. Voice and swallowing changes after thyroidectomy in patients without inferior laryngeal nerve injuries. Surgery. 2006; 140(6):1026–32. discussion 1032-4.

Fig. 1.
PTVD after hemithyroidectomy. There is no international consensus on the diagnosis of PTVD. Various parameters and cut-off values used in previous studies were reviewed and gradual decreasing trend over time was noted [11,12]. PTVD, post thyroidectomy voice disorder; VHI, voice handicap index; GRBAS, grade, roughness, breathiness, asthenia, strain; Pmax, pitch maximum; POD, post operative day.
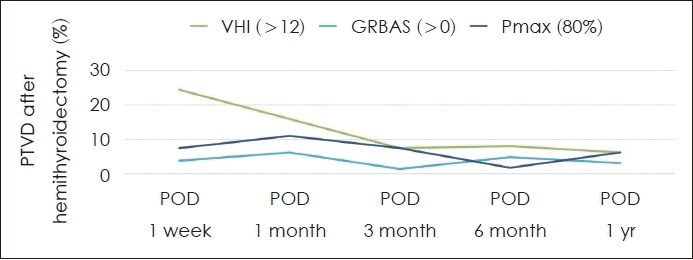
Fig. 2.
Change in objective and subjective voice values from preoperative to 1 year postoperative. VHI is plotted as a solid line with values on the left y-axis, and GRBAS is plotted as a double line with values on the right y-axis (A). Jitter, NHR, and shimmer are plotted as solid lines and represented on the left y-axis, while F0, Pmin, and Pmax are plotted as double lines and represented on the right y-axis (B). VHI, voice handicap index; GRBAS, grade, roughness, breathiness, asthenia, strain; NHR, noise-to-harmony ratio; F0, fundamental frequency; Pmin, pitch minimal; Pmax, pitch maximum.
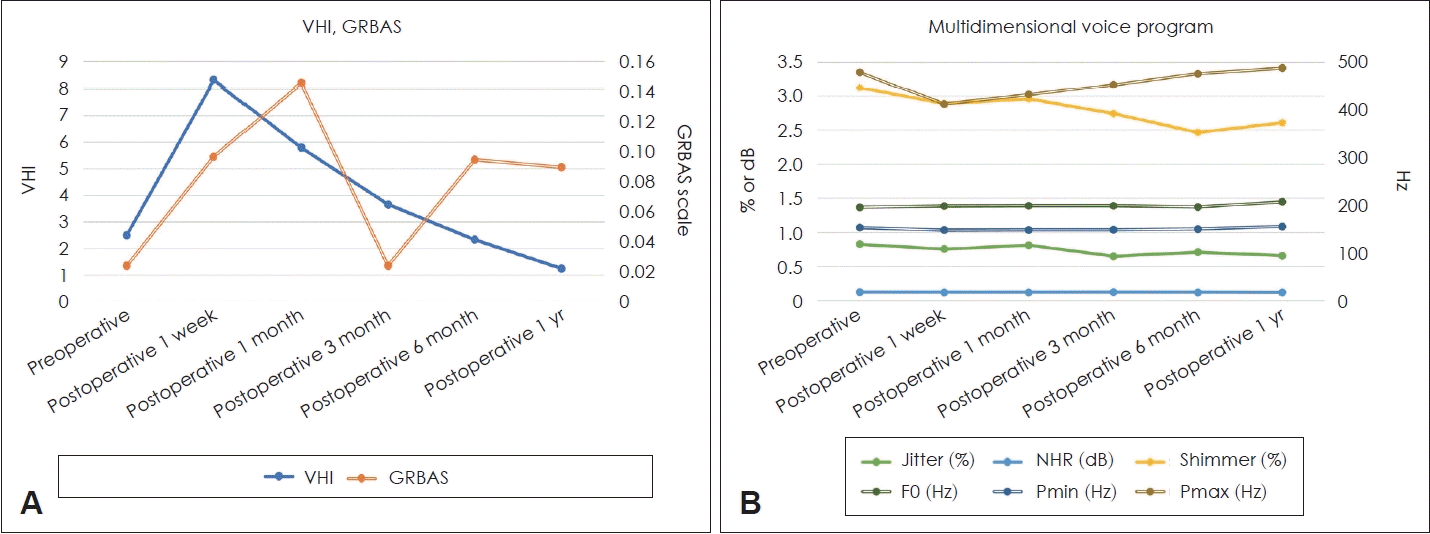
Fig. 3.
Prediction of voice outcomes with skin IONM parameters. Patients were divided into two groups based on whether their ΔV was greater than or less than the median value. Group with ΔV less than the median value are plotted as solid lines and the group with ΔV over the median value as dashed lines. VHI and GRBAS are shown in (A) and (B), Jitter, NHR, and shimmer are shown in (C), and F0, Pmin, and Pmax are shown in (D), respectively. ΔV, difference between V1 and V2; VHI, voice handicap index; GRBAS, grade, roughness, breathiness, asthenia, strain; NHR, noise-to-harmony ratio; F0, fundamental frequency; Pmin, pitch minimal; Pmax, pitch maximum.
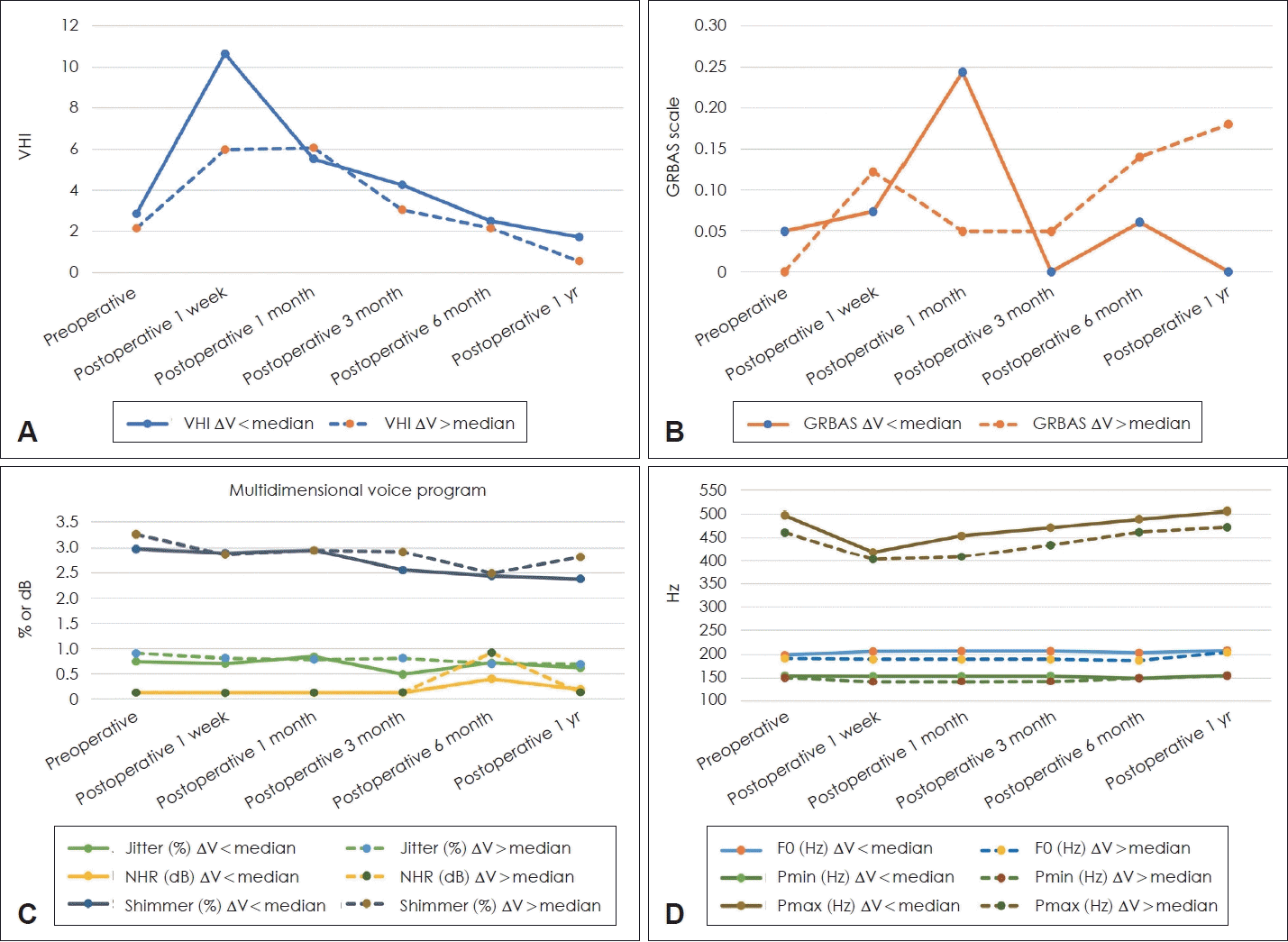
Table 1.
Sequential stimulus location and intensity during hemithyroidectomy using skin electrode intraoperative neuromonitoring using a ball tip probe
Table 2.
Overview of the participants characteristics and pathologic results
Table 3.
Mean, median, and standard deviation of skin electrode intraoperative neuromonitoring values measured during surgery
Table 4.
Change in objective and subjective voice values from preoperative to 1 year postoperative (n=82)
Table 5.
Prediction of voice outcomes with skin IONM parameters




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download