Abstract
Notes
Ethics Statement
This study was approved by the Institutional Review Board of Gyeongsang National University Hospital, and the need for informed consent was waived (IRB No. GNUH 2023-07-033).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author contributions
Conceptualization: JWY. Data Curation: JMN, WJ, JWY. Investigation: JMN, WJ, JWY. Resources: MK, YHC, JSL. Supervision: YHC, JWY. Visualization: JMN, JWY. Writing—original draft preparation: JMN, JWY. Writing—review & editing: JMN, DHS, JWY. Approval of final manuscript: all authors.
ACKNOWLEDGMENTS
References
Fig. 1.
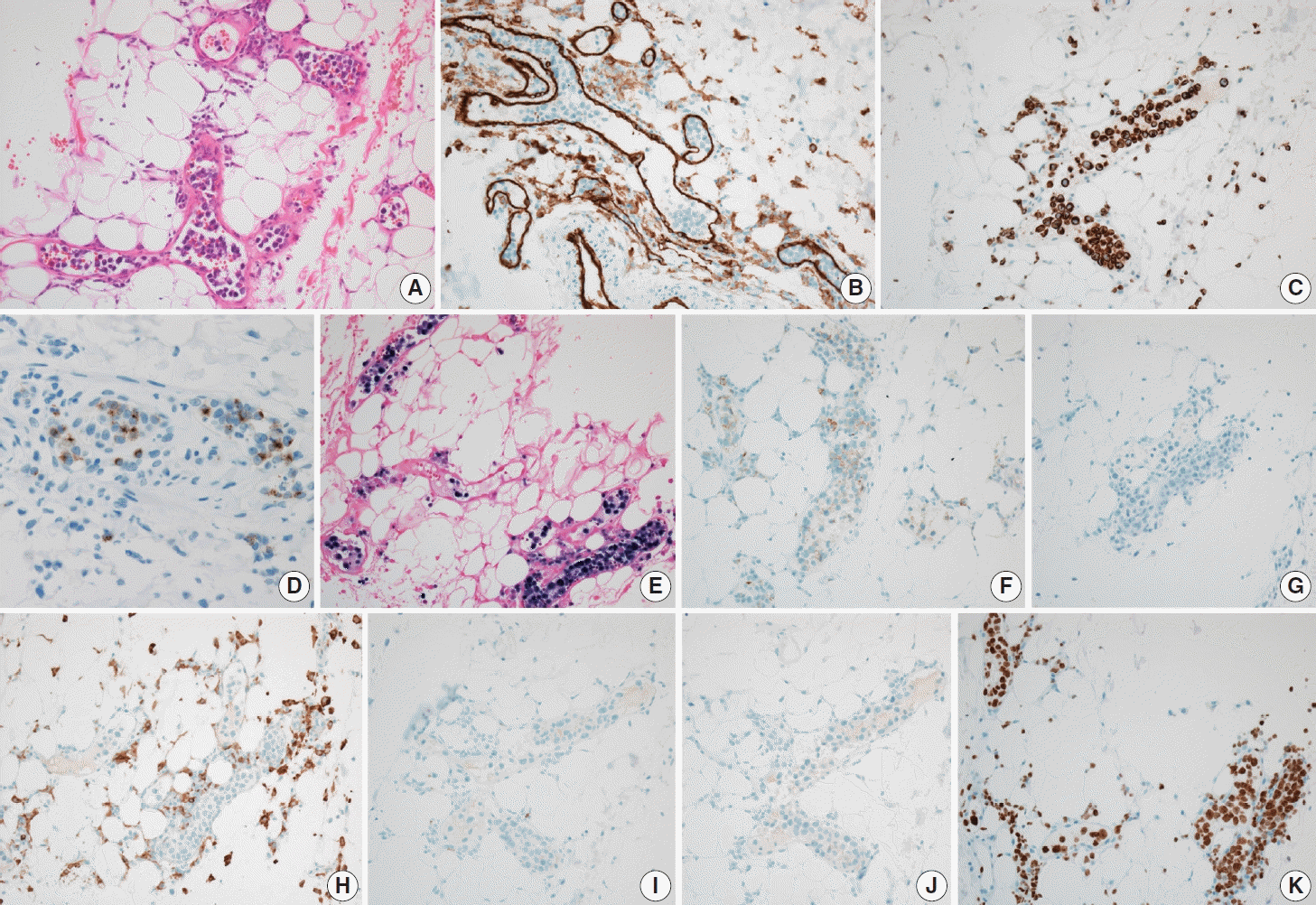
Table 1.
| Case | Age (yr)/Sex | Biopsy sites | Skin lesion | CNS inv. | Cytopenia | Bone marrow inv. | EBV DNA | EBER | TCR | Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Santucci et al. (2003) [4] | 54/M | Skin | + | #NAME? | Leuko- | NA | NA | + | NA | Died 17 mo after dx. |
| Wu et al. (2005) [5] | 41/M | Skin | + | – | – | – | NA | + | Germline | Alive 12 mo after dx. |
| Wu et al. (2005) [5] | 47/F | Skin | + | + | Pan- | + (on IHC) | NA | – | Germline | Died 15 days after dx. |
| Kuo et al. (2006) [6] | 71/F | Skin | + | – | – | – | NA | + | Germline | Alive 5 mo after dx. |
| Song et al. (2007) [7] | 40/F | Skin | + | + | – | – | NA | + | Germline | Alive 7 mo after dx. |
| Nakamichi et al. (2008) [8] | 23/F | Skin | + | – | – | – | NA | + | Germline | Died 9 mo after dx |
| Cerroni et al. (2008) [9] | 67/F | Skin | + | + | – | NA | NA | NA | Clonal (TCRg) | Died 7 days after dx. |
| Cerroni et al. (2008) [9] | 63/M | Skin | + | – | Leuko- | NA | NA | + | Germline | Died 6 mo after dx. |
| Thrombo- | ||||||||||
| Cerroni et al. (2008) [9] | 64/M | Skin | + | NA | – | – | NA | – | Germline | Died 7 mo after dx. |
| Cerroni et al. (2008) [9] | 87/M | Skin | + | – | – | NA | NA | + | Clonal (TCRg) | Died 15 days after dx. |
| Gleason et al. (2008) [10] | 62/M | Skin | + | – | – | – | NA | – | Clonal (TCRg) | Alive 8 mo after dx. |
| Liao (2011) [11] | 42/F | Skin | + | – | – | – | NA | + | NA | Alive 14 mo after dx. |
| Yanning et al. (2013) [12] | 84/F | Skin | + | NA | – | NA | NA | + | Germline | Alive 4 mo after dx. |
| Gbauer et al. (2014) [13] | 72/M | Skin | + | + (6 mo after dx.) | Pan– (6 mo after dx.) | + (40%, 6 mo after dx.) | NA | + | Germline | Died 7 mo after dx. |
| Jang et al. (2014) [14] | 23/F | Skin | + | – | – | – | NA | + | Germline | Died 18 mo after dx. |
| Liu et al. (2014) [15] | 38/F | Skin | + | – (7 mo after dx.) | – | – | NA | + | NA | Died 13 mo after dx. |
| Wang et al. (2015) [16] | 45/M | Skin | + | NA | – | NA | NA | + | Germline | Died 15 days after dx. |
| Wang et al. (2015) [16] | 32/F | Skin | + | – | – | NA | NA | + | Germline | Died 4 mo after dx. |
| Wang et al. (2015) [16] | 18/F | Skin | + | – | – | – | NA | + | Germline | Alive 3 yr after dx. |
| Bi et al. (2015) [17] | 29/M | Skin | + | – | – | – | NA | + | Germline | Died 3 mo after dx. |
| Alhumidi (2015) [18] | 48/F | Skin | + | – | – | – | NA | + | NA | Alive 18 mo after dx. |
| Okonkwo and Jaffe (2017) [19] | 51/M | Skin | + | + | – | NA | NA | + | Clonal (TCRg) | NA |
| Sharma et al. (2017) [20] | 62/F | Brain | – | + | – | + (on TCR) | NA | – | Clonal | Died 7 days after dx. |
| Alegria-Landra et al. (2017) [21] | 81/M | Skin | + | – | – | – | NA | + | Clonal (TCRb & g) | Died 15 days after dx. |
| Zanelli et al. (2018) [22] | 54/M | Autopsy | – | + | Pan- | + (10%) | + (PE & lung) | + | Clonal | Died 18 days after presentation |
| Meisoner et al. (2022) [23] | 53/M | Brain | – | + | – | – | + (CFS) | + | NA | Alive 8 yr after SCT |
| Present case | 67/F | Skin | + | + (sx. only) | Leuko- | – | + (blood) | + | Germline | Died 18 days after dx. |
NK, natural killer; CNS, central nervous system; inv., involvement; EBV, Epstein-Barr virus; EBER, Epstein-Barr virus-encoded small RNA; TCR, T-cell receptor gene rearrangement; M, male; F, female; dx., diagnosis; NA, not available; IHC, immunohistochemistry; PE, pleural effusion; SCT, stem cell transplantation; sx., symptom.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download