Introduction
Materials and Methods
Maintenance of human induced pluripotent stem cells
Generation of hSCOs
Optical clearing and three-dimensional imaging
Microarray
NTDs in vivo with VPA
Immunohistochemistry
Statistical analysis
Ethics approval and consent to participate
Results
Modeling of VPA-induced NTDs with hSCOs
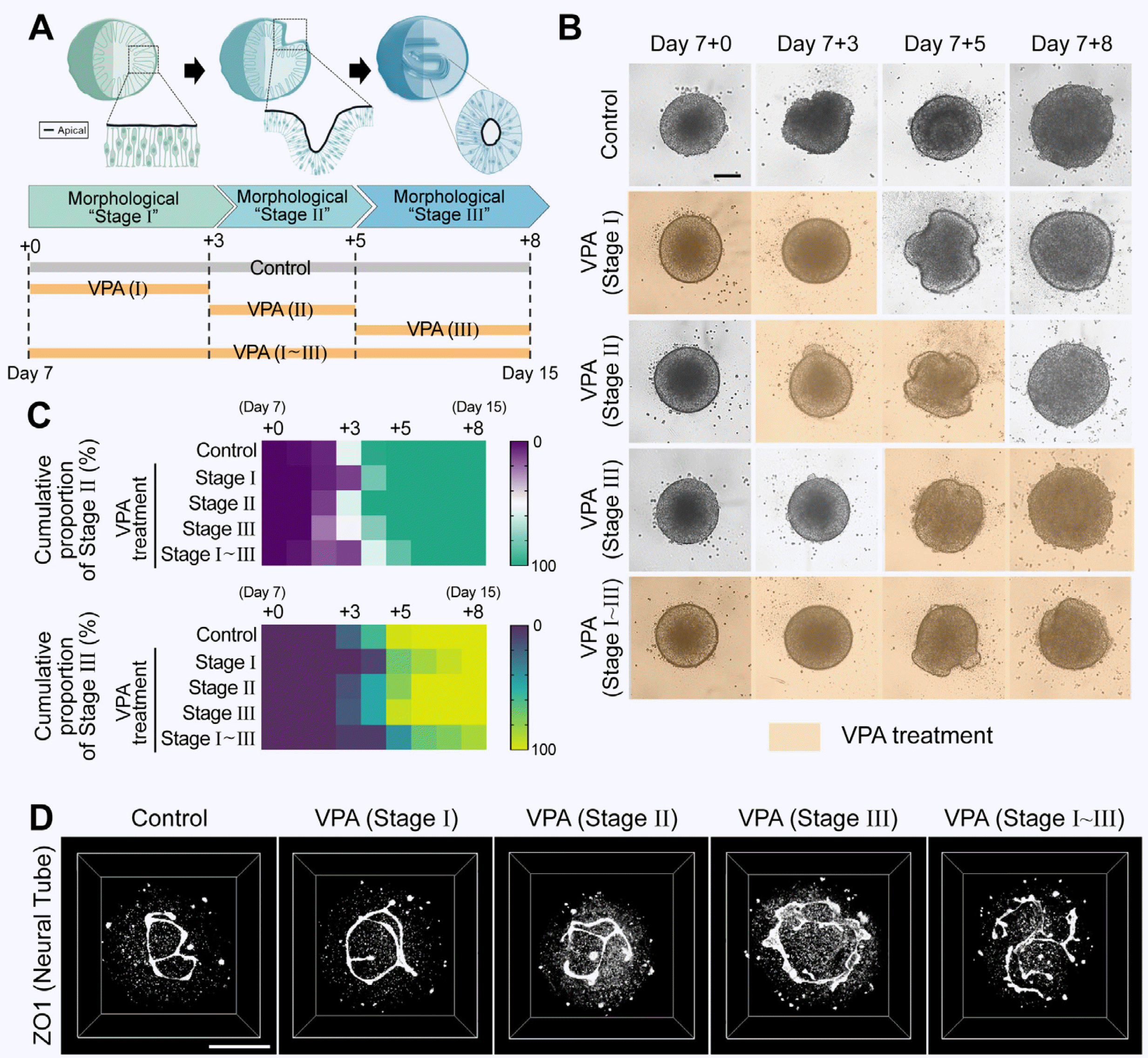 | Fig. 1Modeling of valproic acid (VPA)–induced neural tube defects with human spinal cord organoids (hSCOs). (A) Experimental model to study the effect of duration of VPA treatment on morphogenesis. (B) Re-presentative time-lapse images of neural tube morphogenesis in VPA-treated groups compared with the control. Each image was acquired using an automated imaging system. Scale bar= 200 μm. (C) Quantification of morphogenesis with different durations of VPA treatment. The color of each box indicates the cumulative proportion of neural folding (upper) or neural tube (bottom). Stage of hSCOs at the indicated culture time. (D) Three-dimensional neural tube morphology with different VPA treatment durations on day 15. The structure was visualized using an apical marker and ZO1 staining. Scale bar=100 μm. |
Upregulation of cell–cell junction-related genes in VPA-treated hSCOs
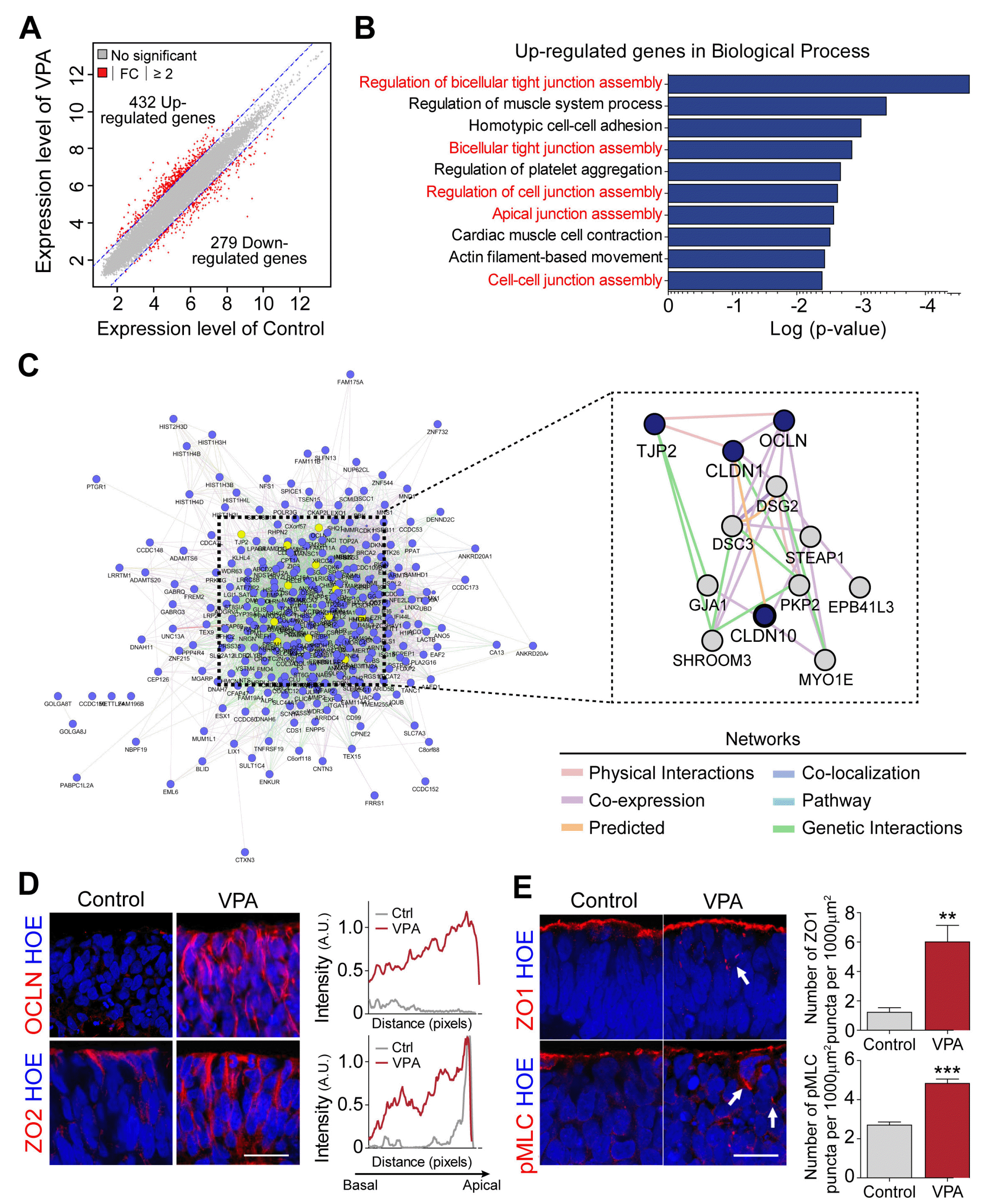 | Fig. 2Transcriptome analysis in human spinal cord organoids (hSCOs) exposed to valproic acid (VPA). (A) Scatter plots of transcriptome levels in hSCOs treated with VPA compared with the controls. Red circles denote the genes whose expression levels were significantly altered (≥2-fold) in VPA-treated vs control groups. (B) Biological processes associated with significantly upregulated genes. (C) Network plot of the upregulated genes enriched in VPA-treated hSCOs. Interactive network is generated using GeneMANIA plugin in Cytoscape. (D) Immunohistochemical evaluation of the upregulation of tight junction proteins, Occludin (OCLN), and ZO2. Average intensity of tight junction proteins was measured along the apico-basal axis. Scale bar=20 μm. (E) Perturbation of polarized localization of ZO1 and pMLC proteins in the neuroepithelial cells of VPA-treated hSCOs. White arrows indicate the ectopic expression of proteins in the VPA-treated group. Bar graphs show the number of ectopic ZO1 and pMLC punctas in neural-plate layers. Unpaired Student’s t-test was used for comparing two groups (**p<0.01 and ***p<0.01). Scale bar=20 μm. FC: fold change, Ctrl: control. |
Effect of tight junction proteins expression and distribution in VPA-induced NTDs
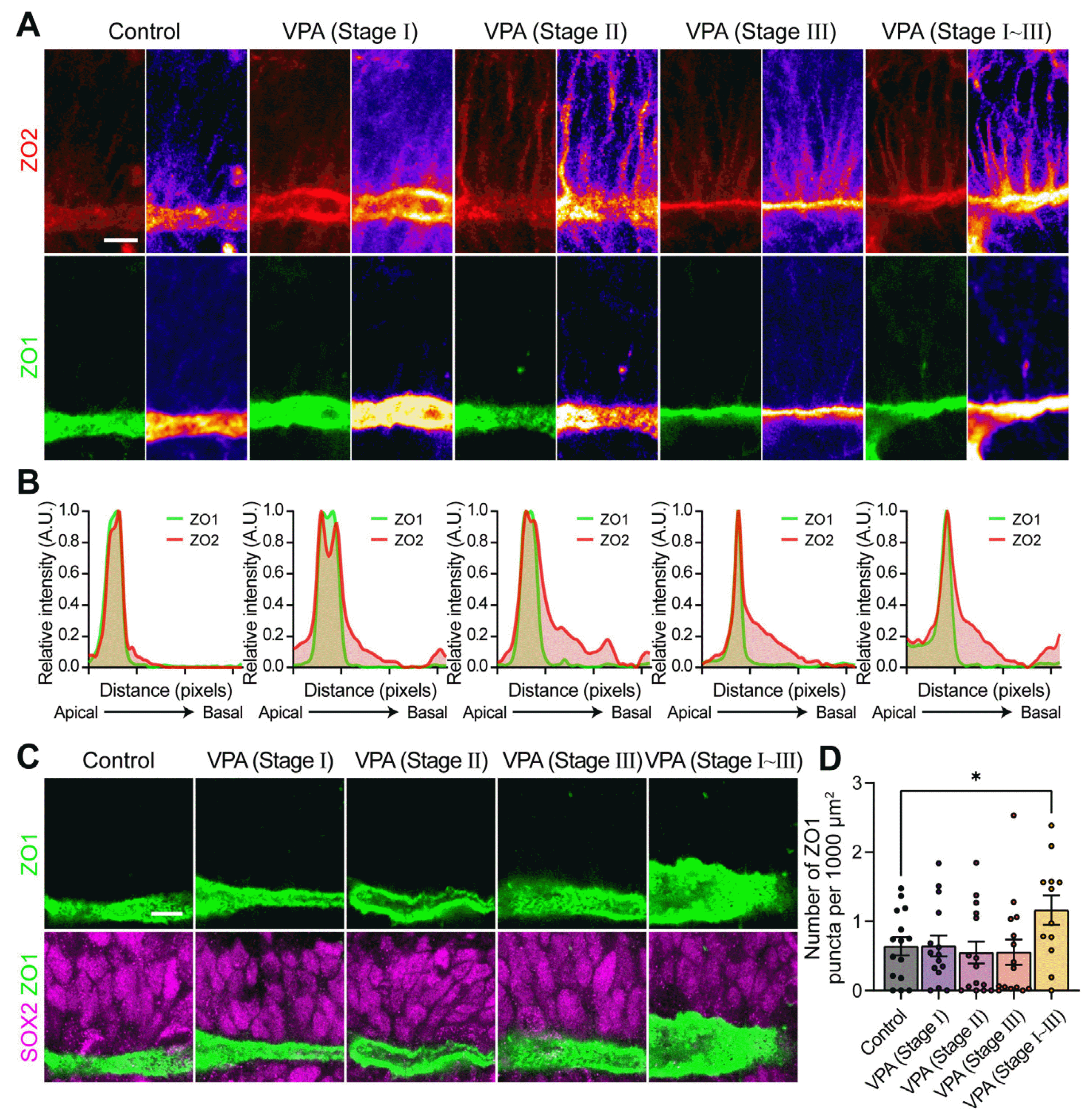 | Fig. 3Effect of tight junction proteins expression/distribution in valproic acid (VPA)-treated human spinal cord or-ganoid. (A) Immunohistochemistry of upregulation of tight junction proteins, ZO2 (red) and ZO1 (green) caused by VPA treatments for different durations. Scale bar=10 μm. (B) Line plots denoting the intensity of ZO1 and ZO2 staining along the closed/opened neural tube. (C) Pertur-bation of polarized localization of ZO1 proteins in neuroepithelium. The neuroepithelial cells were identified as SOX2 (magenta) staining and cellular arrangement surrounding apical marker, ZO1 (green). Scale bar=10 μm. (D) Quantification of the number of ectopic ZO1 punctas within neuroepithelial cell layers. Bar graphs represent mean±SEM, with all the points plotted. Unpaired Student’s t-test was used for comparing two groups (*p<0.05). |
Effects of VPA treatment in vivo using mouse model
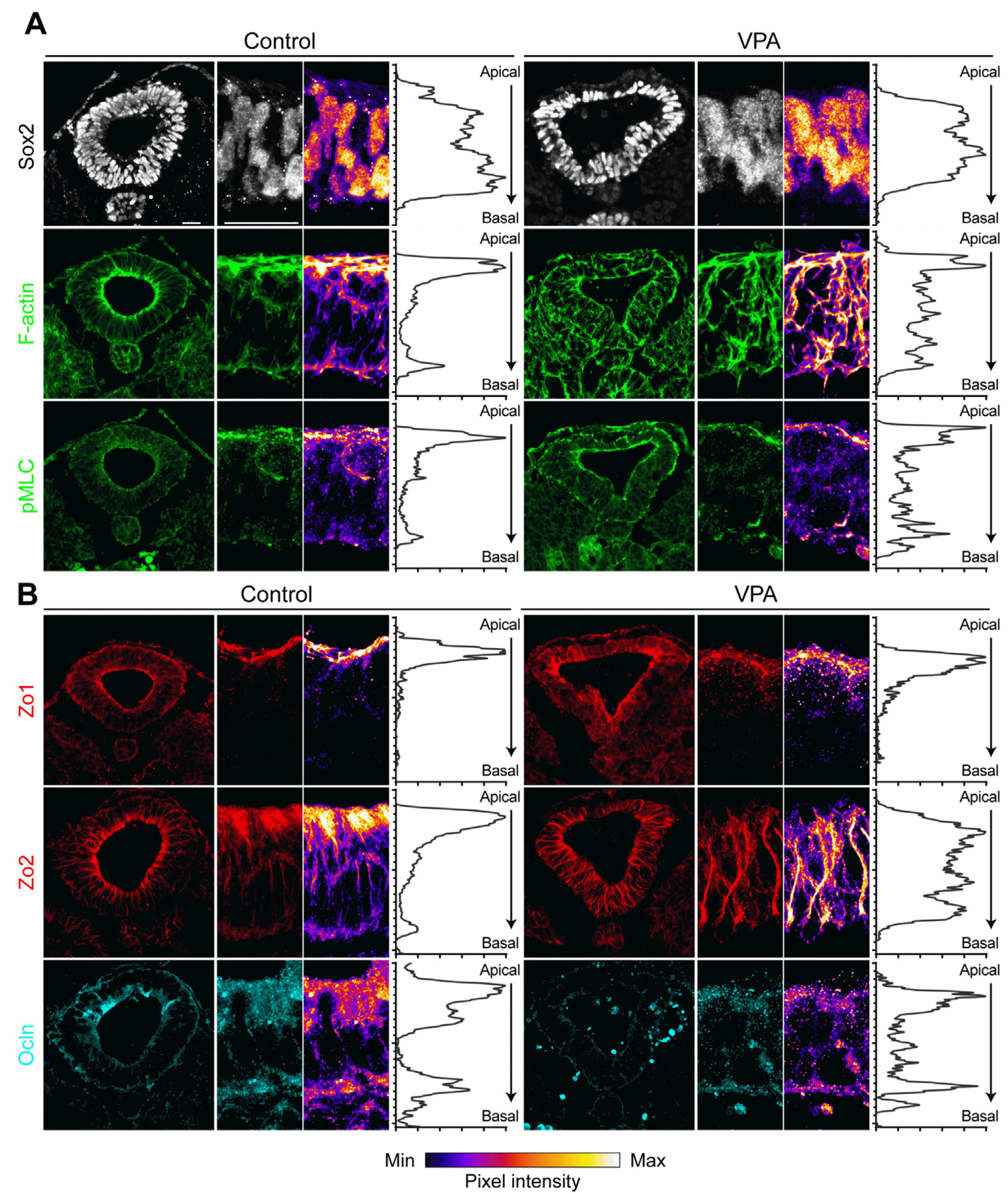 | Fig. 4Effect of valproic acid (VPA) on neural tube in the mouse em-bryo. Immunohistochemistry of VPA-induced neural tube defect mouse models. Various markers were used to observe changes in the expre-ssion/distribution of actin filaments (A, F-actin and pMLC) and tight junction proteins (B, Zo1, Zo2, and Ocln). Dissolved VPA was administrated three times to pregnant female mice at the time of primary neuru-lation. Scale bars=20 μm. Min: minimum, Max: maximum. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download