Abstract
Achalasia, a rare motility disorder of the esophagus, is generally accepted as a premalignant disorder. This paper presents the case of a 72-year-old male with achalasia and synchronous superficial esophageal cancer who experienced dysphagia symptoms for five years. As achalasia is associated with an increased risk of esophageal cancer, both can be treated simultaneously if detected at the time of diagnosis. Achalasia and synchronous esophageal cancer are rarely detected and treated endoscopically. This paper reports a case of concurrent successful treatment.
Achalasia is a rare esophageal motility disorder that leads to difficulty swallowing and other symptoms. It is characterized by loss of esophageal peristalsis and impaired lower esophageal sphincter (LES) relaxation.1 Achalasia is associated with an increased risk of esophageal cancer, as first noted by Fagge2 in 1872. The incidence of esophageal cancer in patients with achalasia is higher than that in the general population, even though the absolute risk is low.3,4 Nevertheless, surveillance endoscopy is not recommended for patients with achalasia owing to this rarity.5
High-resolution manometry (HRM) and minimally invasive peroral endoscopic myotomy (POEM) can assist in diagnosing and treating achalasia. On the other hand, the safety and efficacy of POEM and endoscopic submucosal dissection (ESD) in achalasia with synchronous esophageal cancer have not been investigated.5
This paper reports a case of achalasia with synchronous esophageal cancer that was successfully treated with concomitant ESD and POEM.
A 72-year-old man presented to the emergency department with worsening dysphagia that had started the previous day. He was administered amlodipine for hypertension, teneligliptin and metformin for type II diabetes mellitus, and ezetimibe and rosuvastatin for dyslipidemia. He had experienced recurrent dysphagia for five years and was referred to the authors’ department for dysphagia when he had been hospitalized for empyema three years prior. The symptoms persisted for two months. Therefore, HRM was performed, and the patient was diagnosed with type I achalasia. He was treated with pneumatic balloon dilation; his symptoms improved, and he was followed up on an outpatient basis.
Despite outpatient follow-up, the patient's dysphagia symptoms had recently worsened, prompting a visit to the emergency department for a comprehensive evaluation to determine the underlying cause. A Chest CT on the day of hospital admission revealed a dilated esophagus and residual food. Upper gastrointestinal endoscopy was performed the following day, but a detailed observation was not possible because of the food residue (Fig. 1A). After fasting for two days, upper GI endoscopy was repeated, and the food residue was removed endoscopically (Fig. 1B, D). Subsequently, a detailed upper GI endoscopy revealed a well-demarcated, erythematous depressed lesion, approximately 15 mm in size, at the posterior side of 33cm from the incisor (Figs. 1C, 2A), along with vascular changes on the narrow band image (Fig. 2B). A histology examination confirmed the carcinoma. Subsequently, HRM was performed and showed elevated integrated relaxation pressure (IRP) over 15 mmHg. No contractile activity of the esophageal body was identified, indicating type I achalasia (Fig. 3A). A timed barium esophagogram revealed a bird's beak sign on the esophagogastric junction, a dilated esophageal body, and a sigmoid- like appearance (Fig. 3C). Esophageal transit scintigraphy demonstrated a prolonged esophageal transit time with 99% of the food remaining after 10 minutes (Fig. 3B).
Neck CT, abdominal and pelvic CT, and PET-CT were performed to evaluate superficial esophageal cancer and type I achalasia. No metastatic findings were observed. Endoscopic ultrasonography revealed cancer confined to the mucosa and mild thickening of the esophageal smooth muscle layer.
After consultation with the patient and his family, ESD for esophageal cancer (Fig. 2) and POEM for achalasia (Fig. 4) were performed simultaneously. First, ESD was performed at 32–35 cm from the incisor, and the resected specimen was approximately 3 cm in diameter. Subsequently, a mucosal incision for the POEM entry site was made at 39 cm from the incisors. Submucosal tunneling continued to the gastric cardia, which was 48 cm from the incisors, and myotomy was performed from 42 cm to 48 cm from the incisors without perforation around the LES (4 cm on the esophagus side and 2 cm on the gastric cardia side). The ESD and POEM run times were 61 and 49 minutes, respectively. Esophagography was per formed to assess the POEM opening site and esophagogastric junction for leakage; no abnormalities were found. The patient was discharged without any other complications after the diet was started. Subsequent histology confirmed a well-to-poorly differentiated squamous cell carcinoma (11 mm×8 mm) with high-grade dysplasia (10 mm×9 mm) confined to the lamina propria of the mucosal layer with clear margins. Despite discussing additional treatment because of the poor differentiation, the pathological review revealed only a small portion of poorly differentiated tissue. In addition, the patient and his family refused further therapeutic interventions. Consequently, the patient underwent regular follow-up with endoscopy and other imaging modalities. The patient was followed up for one year with no recurrence of esophageal cancer, increased IRP on HRM, or symptoms of dysphagia.
Achalasia is caused by the loss of the inhibitory ganglion in the myenteric plexus of the esophagus. In the initial stage, degeneration of the inhibitory nerves results in the unopposed action of excitatory neurotransmitters, resulting in non-peristaltic contractions. The progressive loss of cholinergic neurons results in dilation and a lower contraction amplitude in the esophageal body.6
Oral pharmacological therapy, botulinum toxin injection, pneumatic balloon dilatation, POEM, and laparoscopic Heller’s myotomy provide effective results for achalasia by relieving symptoms, such as dysphagia and related complications. Laparoscopic Heller’s myotomy is a first-line treatment for achalasia that can achieve excellent outcomes but is invasive. Recently, POEM has gained popularity as a commonly used treatment for achalasia. Recent guidelines show that POEM has similar outcomes to laparoscopic Heller’s myotomy.7
In patients with suboptimally treated or untreated achalasia, food residue and fluid in the esophageal body can result in bacterial overgrowth and chemical irritation. This leads to chronic hyperplastic esophagitis and malignant transformation of esophageal epithelial cells into esophageal squamous cell carcinoma.4 In addition, effective treatment of achalasia can lead to sphincter insufficiency, resulting in gastroesophageal reflux disease and Barrett’s esophagus (BE). Esophageal adenocarcinoma can develop after BE but is less common than squamous cell carcinoma.3
As a modality for superficial esophageal cancer, ESD has evolved into a viable treatment because of its equivalent long-term survival outcomes to surgical resection.8,9 The simultaneous detection and endoscopic treatment of achalasia and esophageal cancer is rare. Few cases where achalasia and superficial esophageal cancer were treated simultaneously have been reported (Table 1).10,11 In one case, concurrent chemoradiotherapy was performed on a patient with achalasia and advanced esophageal cancer.12 In other cases, ESD for esophageal cancer was performed either before or after POEM for achalasia rather than at the same time.5 This paper reports the successful endoscopic treatment of concomitant achalasia and superficial esophageal cancer.
In a multicenter study in Japan by Sato et al.5, multivariate analysis revealed the risk factors for the development of esophageal cancer in achalasia, including disease duration, age at diagnosis, male sex, alcohol consumption, smoking status, and sigmoid-type achalasia. Of 43 esophageal cancer cases, 31 (72.1%) were identified during screening for a one-year follow-up after POEM. Regarding an endoscopic resection for superficial esophageal cancer, the en-bloc resection rate for cases in which cancer was detected before POEM was 95.8% (46/48), and 89.3% (25/28) for cases detected after; these results were not significant (p=0.351). Hence, ESD is feasible for achalasia, even though it is challenging along the POEM-line, as three patients had esophageal cancer on the POEM-line. Two patients underwent ESD. One underwent a piecemeal resection because of submucosal fibrosis, and the other underwent surgery because ESD was difficult due to severe fibrosis.5
Therefore, performing ESD and POEM simultaneously may reduce the burden of general endotracheal anesthesia (GETA) procedures twice13 and reduce the risk of fibrosis resulting from performing ESD or POEM first. Achalasia and esophageal cancer can be treated concurrently if they are located in different regions. In the present case, the esophageal cancer lesion was determined to be in the middle of the esophagus (33 cm from the incision in the dilated esophagus), and the morphology of the achalasia was a stretched and rotated sigmoid. Hence, the opening for the ESD and POEM was more than 4 cm apart. Consequently, ESD was feasible without any hindrance to the POEM procedure. On the other hand, depending on the priority and symptoms between the two treatments, sequential treatment should be considered if the lesion is located in an area that may affect the POEM procedure. In addition, other options, such as chemoradiotherapy for esophageal cancer in conjunction with POEM, may also be considered.
This paper reports a case in which superficial esophageal cancer was identified at the time of achalasia diagnosis and was treated successfully with simultaneous ESD and POEM. Although achalasia increases the risk of esophageal cancer, endoscopic surveillance is controversial regarding its cost-effectiveness.14 On the other hand, regular screening for esophageal cancer is essential for patients with achalasia.15 Food retention is a known risk factor for esophageal cancer in patients with achalasia. Therefore, it is essential to thoroughly remove the food and closely monitor the esophageal mucosa from diagnosis.
REFERENCES
1. Vaezi MF, Pandolfino JE, Vela MF. 2013; ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 108:1238–1249. quiz 1250DOI: 10.1038/ajg.2013.196. PMID: 23877351.

2. Fagge CH. 1872; A case of simple stenosis of the oesophagus, followed by epithelioma. Guy's Hosp Report (London). 17:413.
3. Sato H, Terai S, Shimamura Y, et al. 2021; Achalasia and esophageal cancer: a large database analysis in Japan. J Gastroenterol. 56:360–370. DOI: 10.1007/s00535-021-01763-6. PMID: 33538893.

4. Leeuwenburgh I, Haringsma J, Van Dekken H, Scholten P, Siersema PD, Kuipers EJ. Long-term risk of oesophagitis, Barrett's oesophagus and oesophageal cancer in achalasia patients. Scand J Gastroenterol Suppl. 2006; (243):7–10. DOI: 10.1080/00365520600664201. PMID: 16782616.

5. Sato H, Nishikawa Y, Abe H, et al. 2022; Esophageal carcinoma in achalasia patients managed with endoscopic submucosal dissection and peroral endoscopic myotomy: Japan Achalasia Multicenter Study. Dig Endosc. 34:965–973. DOI: 10.1111/den.14197. PMID: 34787940.

6. Ghoshal UC, Daschakraborty SB, Singh R. 2012; Pathogenesis of achalasia cardia. World J Gastroenterol. 18:3050–3057. DOI: 10.3748/wjg.v18.i24.3050. PMID: 22791940. PMCID: PMC3386318.

7. Jung HK, Hong SJ, Lee OY, et al. 2020; 2019 Seoul consensus on esophageal achalasia guidelines. J Neurogastroenterol Motil. 26:180–203. DOI: 10.5056/jnm20014. PMID: 32235027. PMCID: PMC7176504.

8. Aadam AA, Abe S. 2018; Endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 31:doy021. DOI: 10.1093/dote/doy021. PMID: 29982386.

9. Das A, Singh V, Fleischer DE, Sharma VK. 2008; A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 103:1340–1345. DOI: 10.1111/j.1572-0241.2008.01889.x. PMID: 18510606.

10. Tang X, Ren Y, Jiang B, Gong W. 2015; Education and imaging: Gastroenterology: Endoscopic mucosal resection for esophageal dysplasia in an achalasia patient followed by peroral endoscopic myotomy. J Gastroenterol Hepatol. 30:1563. DOI: 10.1111/jgh.13034. PMID: 26177680.

11. Shi S, Fu K, Dong XQ, Hao YJ, Li SL. 2017; Combination of concurrent endoscopic submucosal dissection and modified peroral endoscopic myotomy for an achalasia patient with synchronous early esophageal neoplasms. World J Gastrointest Endosc. 9:99–104. DOI: 10.4253/wjge.v9.i2.99. PMID: 28250904. PMCID: PMC5311480.

12. Park JC, Lee YC, Kim SK, et al. 2009; Achalasia combined with esophageal cancer treated by concurrent chemoradiation therapy. Gut Liver. 3:329–333. DOI: 10.5009/gnl.2009.3.4.329. PMID: 20431771. PMCID: PMC2852741.

13. Goudra B, Saumoy M. 2022; Anesthesia for advanced endoscopic procedures. Clin Endosc. 55:1–7. DOI: 10.5946/ce.2021.236. PMID: 34974678. PMCID: PMC8831399.
14. Leeuwenburgh I, Scholten P, Alderliesten J, et al. 2010; Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol. 105:2144–2149. DOI: 10.1038/ajg.2010.263. PMID: 20588263.

15. Han SY, Youn YH. 2023; Role of endoscopy in patients with achalasia. Clin Endosc. 56:537–545. DOI: 10.5946/ce.2023.001. PMID: 37430397. PMCID: PMC10565433.

Fig. 1
Pre-procedure studies. (A) High-resonance manometry (HRM). HRM indicated type I achalasia owing to the absence of peristalsis, no contractile activity, and no pressurization within the esophageal body. (B) Dynamic esophageal scintigraphy showing prolongation of esophageal transit time. (C) Barium esophagography reveals a “bird-beak” appearance of the esophagogastric junction, with a dilated esophageal body and sigmoid-like appearance.
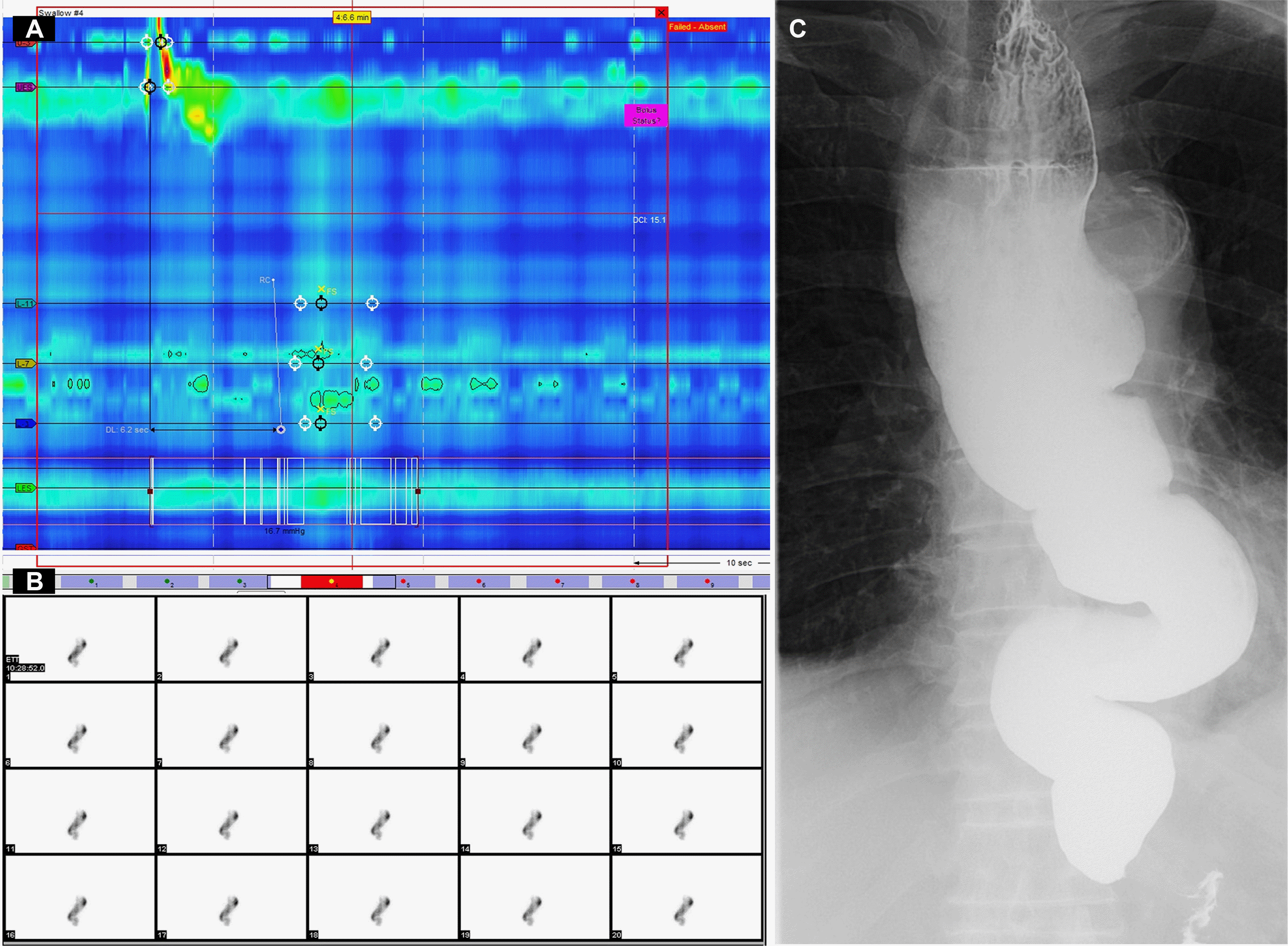
Fig. 2
Endoscopic findings. (A) Dilated esophagus drooping to both sides of the spine. (B) The narrowing of the distal esophagus is noted with food retention. (C) A 15 mm sized, hyperemic depressed lesion with vascular abnormality suspicious for esophageal cancer is observed 33 cm from the upper incisor. (D) Endoscopic food residue removal was performed.
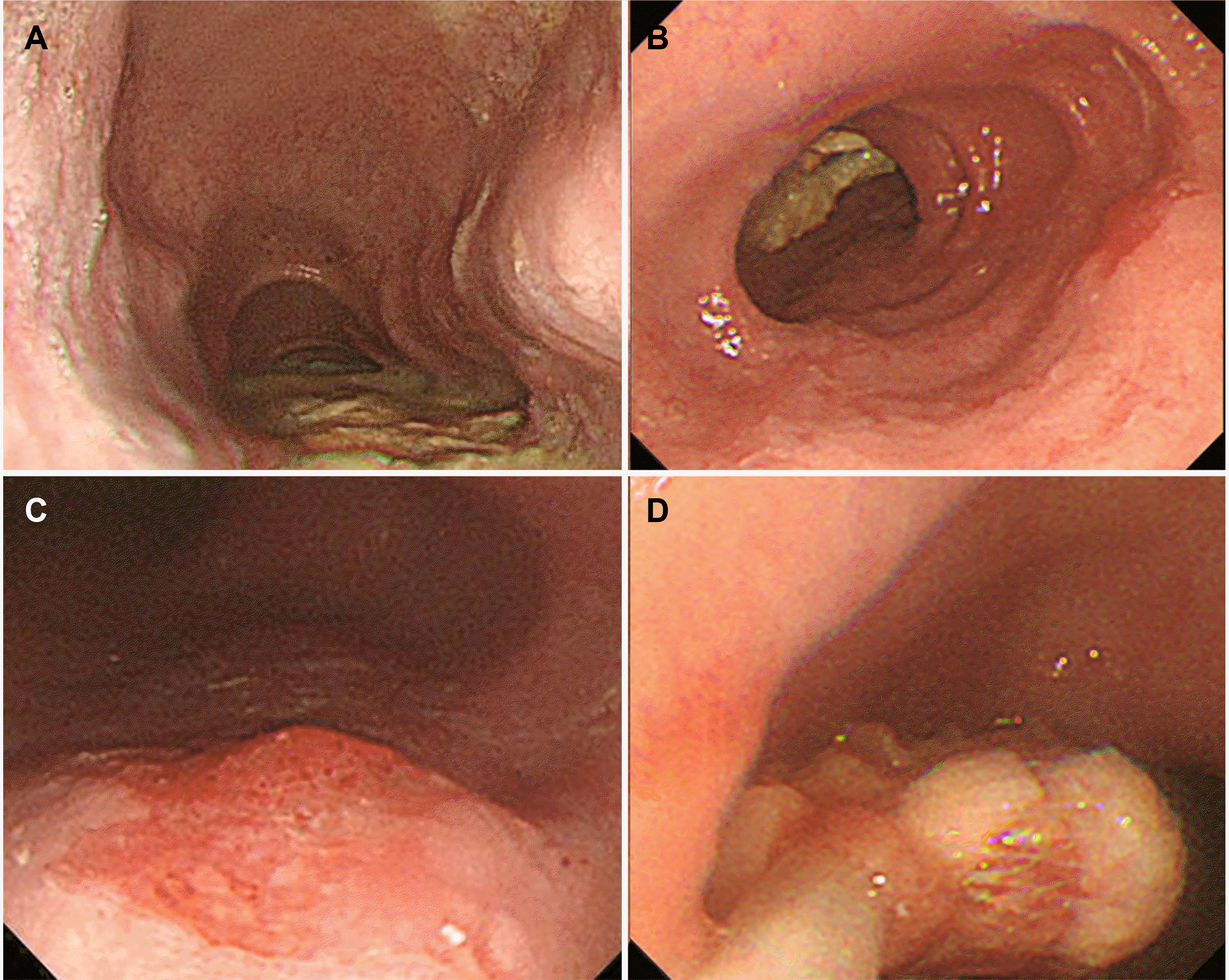
Fig. 3
Endoscopic submucosal dissection (ESD) for esophageal superficial cancer. (A) White light image view of the lesion. (B) The narrow banding image endoscopy revealed a demarcated line, indicating the lesion margins as a brownish area. (C) The lesion was evaluated using Lugol’s solution (iodine), revealing no iodine uptake. (D) After marking and pre-cut, submucosal dissection was performed. (E) En-bloc resection was achieved, and the post-ESD scar was present on endoscopy.
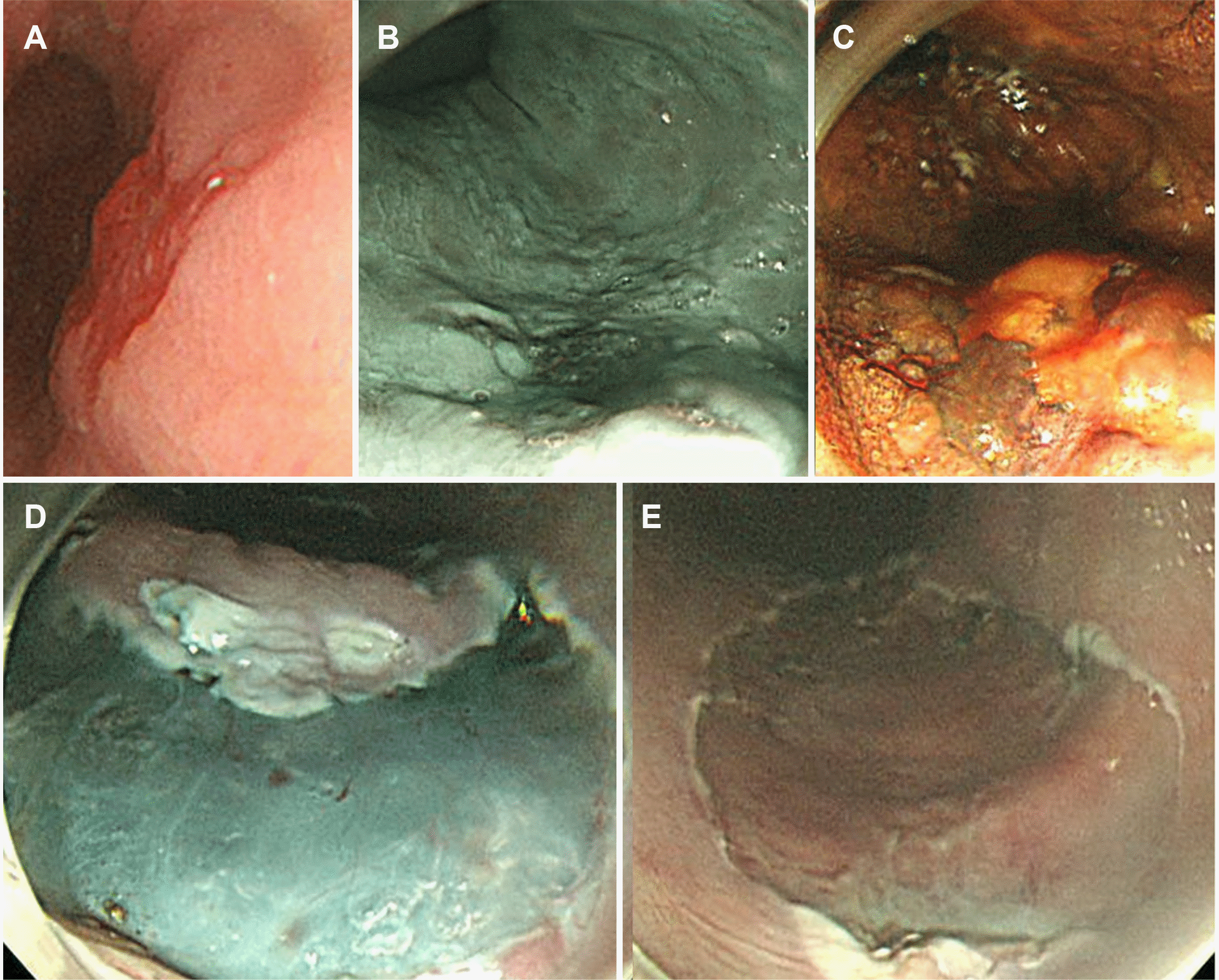
Fig. 4
Peroral endoscopic myotomy for achalasia. (A) and (B) mucosal injections. (C) A submucosal tunnel is established. (D) The submucosa is dissected distally along the muscular layer. (E) Myotomy is performed. (F) The mucosal entry was closed with clipping.
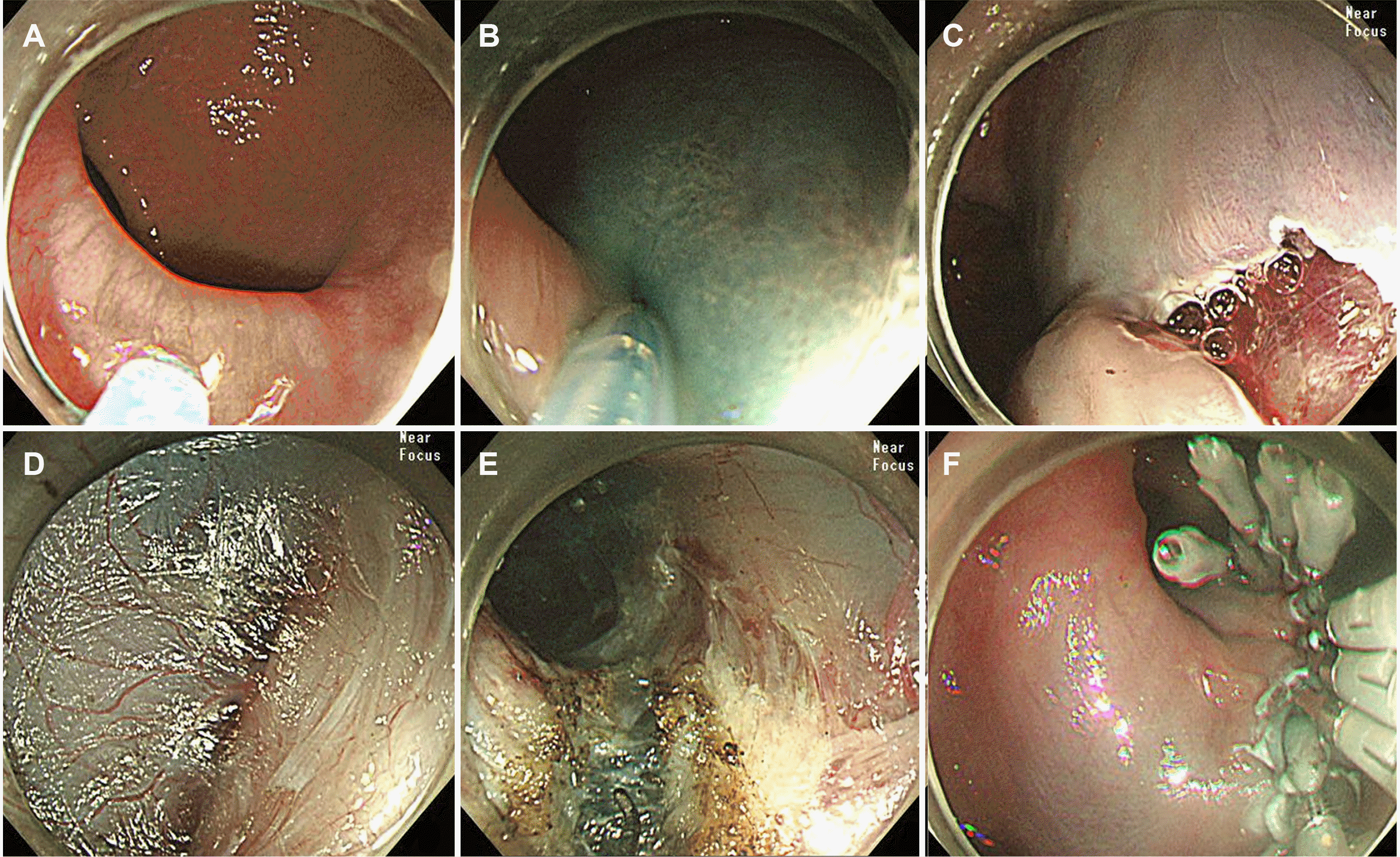
Table 1
Cases of Synchronous Esophageal Cancer with Achalasia and Their Treatment
| No. | First author (yr) | Age (yr)/Sex | Endoscopic finding of esophageal lesion | Histopathology | Type of achalasia | Procedure |
|---|---|---|---|---|---|---|
| 1 | Tang et al.10 (2015) | 41/Male | Anterior part of the middle esophagus - IPCL V1 | Low-grade dysplasia | Unknown | Concurrent EMR and POEM |
| 2 | Shi et al.11 (2017) | 50/Male | At the UI 32 cm, 1.0×0.8 cm sized - IPCL V1 | High-grade dysplasia | Sigmoid | Concurrent ESD and POEM |
| At the UI 30 cm, 1.0×1.5 cm sized - IPCL IV-V1 | Low-grade dysplasia | |||||
| 3 | Park et al.12 (2009) | 51/Male |
At the UI 42 cm, fungating, exophytic mass with an irregular and friable surface involving 90% of the luminal circumference |
Squamous cell carcinoma | Straight | Concurrent chemoradiation therapy |
| 4 | Our case (2023) | 72/Male | At the UI 33 cm, 15 mm sized, hyperemic depressed lesion with vascular abnormality | Squamous cell carcinoma | Sigmoid | Concurrent ESD and POEM |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download