INTRODUCTION
In recent years, demands for improving facial asymmetry along with esthetic perception have increased. Improvement in facial asymmetry is attributed to an in-depth diagnostic analysis of underlying hard tissue and facial soft tissue.
1-3 With the aid of current three-dimensional (3D) imaging techniques, such as cone-beam computed tomography (CBCT) and facial scanning, precise diagnostic data can be obtained to analyze the hard and soft tissue features of patients.
4,5 Notably, as perceived facial asymmetry mostly depends on soft tissue features, treatment success relies on the soft tissue outcome. Therefore, a sufficient understanding of the soft tissue deviation relative to skeletal asymmetry is crucial to ensure high treatment predictability.
The prevalence of asymmetry is higher in skeletal Class III patients than in Class I or II patients.
6-8 Considering the fact that the mandible is affected in 74% of facial asymmetry patients,
6 the influence of mandibular asymmetry on the facial soft tissue might be crucial. As the asymmetric mandibles have different amounts of yaw and roll rotations and related morphologic alterations, the facial soft tissue could be affected accordingly. However, previous studies that have reported soft tissue menton (Me’) deviation, transverse cant or lateral deviation of the lips, and nasal deviation were only based on a single asymmetry group.
9-12 Because of this limitation, it would be difficult to elaborate on which part of soft tissue deviations is closely associated with a certain deviation of the underlying bone.
By grouping different asymmetry types, previous studies have effectively revealed the respective traits of dental compensation in each asymmetry type.
13,14 Mandibular asymmetry can be clearly described using 3D rotations, such as roll and yaw, or translation, as suggested by Ackerman et al.
15 These terminologies are commonly used to indicate exact dentofacial positions, whereas several new specific classifications of mandibular asymmetry have been reported in other previous studies,
13,16,17 thus enhancing our understanding of the relationship between hard and soft tissue deviations. Meanwhile, regarding methodologies of soft tissue analysis, earlier studies mainly measured the deviation of the lips or Me’ position but rarely measured the deviation in other areas, such as the nose, nasolabial fold, and chin contour.
10,11,18,19 In addition, the soft tissues were quantified three-dimensionally using the volume or thickness of multiple grid-based areas in other previous studies;
1,3,20 however, the averaged values of the areas might obscure precise measurements on specific landmarks. These limited data may lead to low predictability of soft tissue changes and insufficient improvement in facial asymmetry after surgery. In other words, by measuring soft tissue vertically, transversely, and anteroposteriorly with more specific landmarks, the 3D soft tissue deviation patterns related to each mandibular asymmetry type would be clearly obtained and easily understood by clinicians, and this information can be used to predict the soft tissue responses of each soft tissue after surgical skeletal movement of different mandibular asymmetry types.
Therefore, this study aimed to measure the 3D soft tissue deviation of the nose, lips, and chin in Class III patients with roll-, yaw-, and translation-dominant mandibular asymmetries. The soft tissue measurements were also assessed to determine their correlation with the underlying skeletal and dental measurements. The null hypothesis was that there would be no significant difference in soft tissue deviations between the different mandibular asymmetry types.
Go to :

MATERIALS AND METHODS
This study was approved by the Institutional Review Board at Kyungpook National University Dental Hospital (KNUDH-2022-12-03-00).
The sample size was determined using G*power (version 3.1.9.7; Heinrich Heine University of Düsseldorf, Düsseldorf, Germany) with reference to previous CBCT research on soft tissue analysis.
4 The appropriate sample size was calculated to be 28 patients in each group, with a test power of 0.80, a two-sided significance level of 0.05, and an effect size of 0.78. To increase the power of this study, 30 patients were enrolled in each group.
The inclusion criteria were as follows: patients with skeletal Class III relationship (point A-nasion-point B angle [ANB] < 0°), moderate-to-severe facial asymmetry (> 4 mm menton [Me] deviation relative to the midsagittal plane [MSP]),
21,22 and no or mild dental crowding (tooth size-arch length discrepancy < 3 mm). The exclusion criteria were as follows: patients with congenital missing teeth except for the third molars, dental spacing, dental prosthesis, and a history of previous orthodontic treatment, orthognathic surgery, or craniofacial syndrome and/or trauma.
The total sample group included 90 patients (59 males and 31 females; age, 21.75 ± 2.78 years; age range, 18–37.9 years) who underwent an orthodontic diagnosis using CBCT scans at Kyungpook National University Dental Hospital, Daegu, Korea, between January 2010 and December 2018. Cone beam computed tomography scans (120 kVp, 15 mA, 19-cm field of view, 0.377 mm voxel size, 9.6-s scan time) were acquired using a CT scanner (CB MercuRay, Hitachi, Osaka, Japan), and variables were measured using the Invivo 6 anatomy imaging software (Anatomage, San Jose, CA, USA).
The enrolled patients were classified into three asymmetry groups according to the extent of mandibular rolling (the angle formed by the mandibular horizontal plane [MHP] to Frankfort horizontal plane [FHP]), yawing (the angle formed by the mandibular midsagittal plane [MnMSP] to MSP), and translation (the distance between the midpoint of bilateral mental foramen [MFmid] and MSP) (
Figure 1).
22 The roll-dominant group consisted of patients with > 5° mandibular rolling and < 3° mandibular yawing; the yaw-dominant group, patients with > 5° mandibular yawing and < 3° mandibular rolling; and the translation-dominant group, patients with < 3° mandibular rolling and yawing and > 4 mm MFmid deviation relative to MSP.
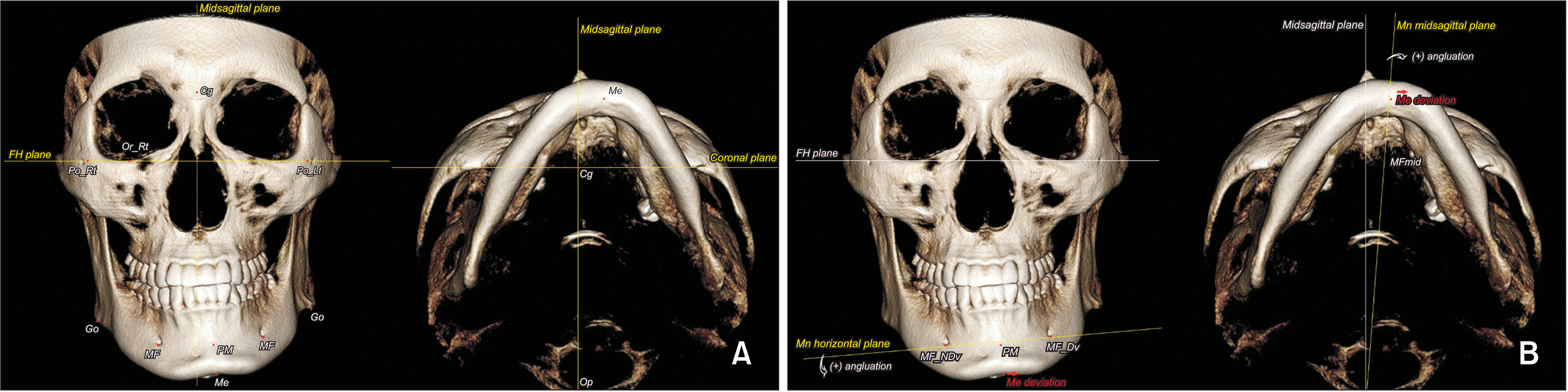 | Figure 1
A, Skeletal landmarks and cranial reference planes. B, Mandibular rolling and yawing based on the mandibular reference planes.
FH, Frankfort horizontal; Cg, crista galli; Or, orbitale; Po, porion; Go, gonion; MF, mental foramen; PM, protuberance menti; Me, menton; MFmid, midpoint of the bilateral MF; NDv, nondeviated side; Dv, deviated side; Mn, mandibular; Rt, right; Lt, left; Op, opisthion.

|
Cephalometric variables were measured to investigate the sagittal and vertical skeletal relationships in each group.
Definitions of soft tissue, skeletal, and dental landmarks and variables used in this study are described in
Tables 1 and
2, and
Figures 1–
4.
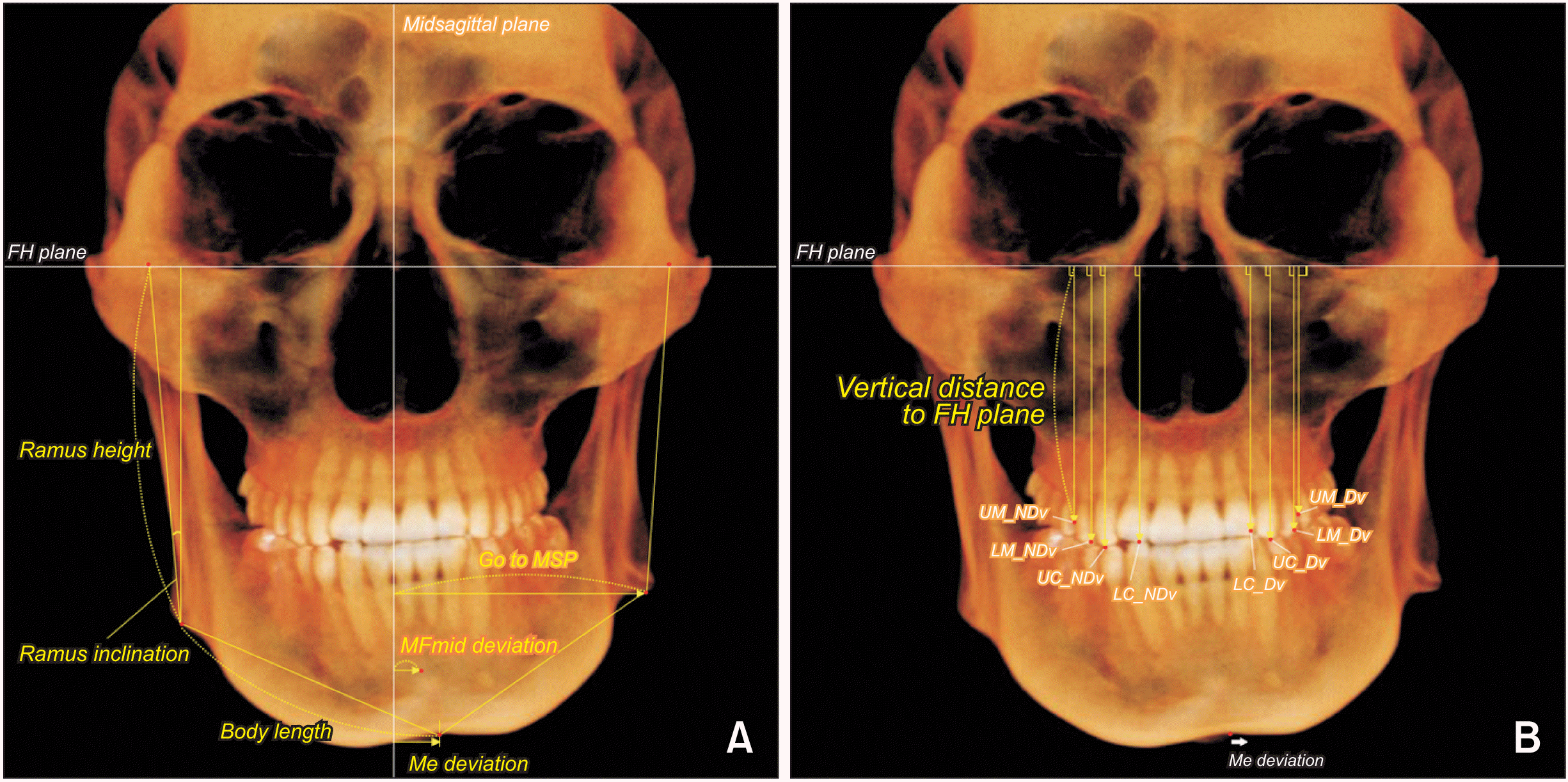 | Figure 2
Skeletal and dental measurements. A, Skeletal distance and angulation. B, Dental vertical distance.
FH, Frankfort horizontal; Go, gonion; MSP, midsagittal plane; MFmid, midpoint of bilateral mental foramen; Me, menton; UM, maxillary first molar; NDv, nondeviated side; LM, mandibular first molar; UC, maxillary canine; LC, mandibular canine; Dv, deviated side.

|
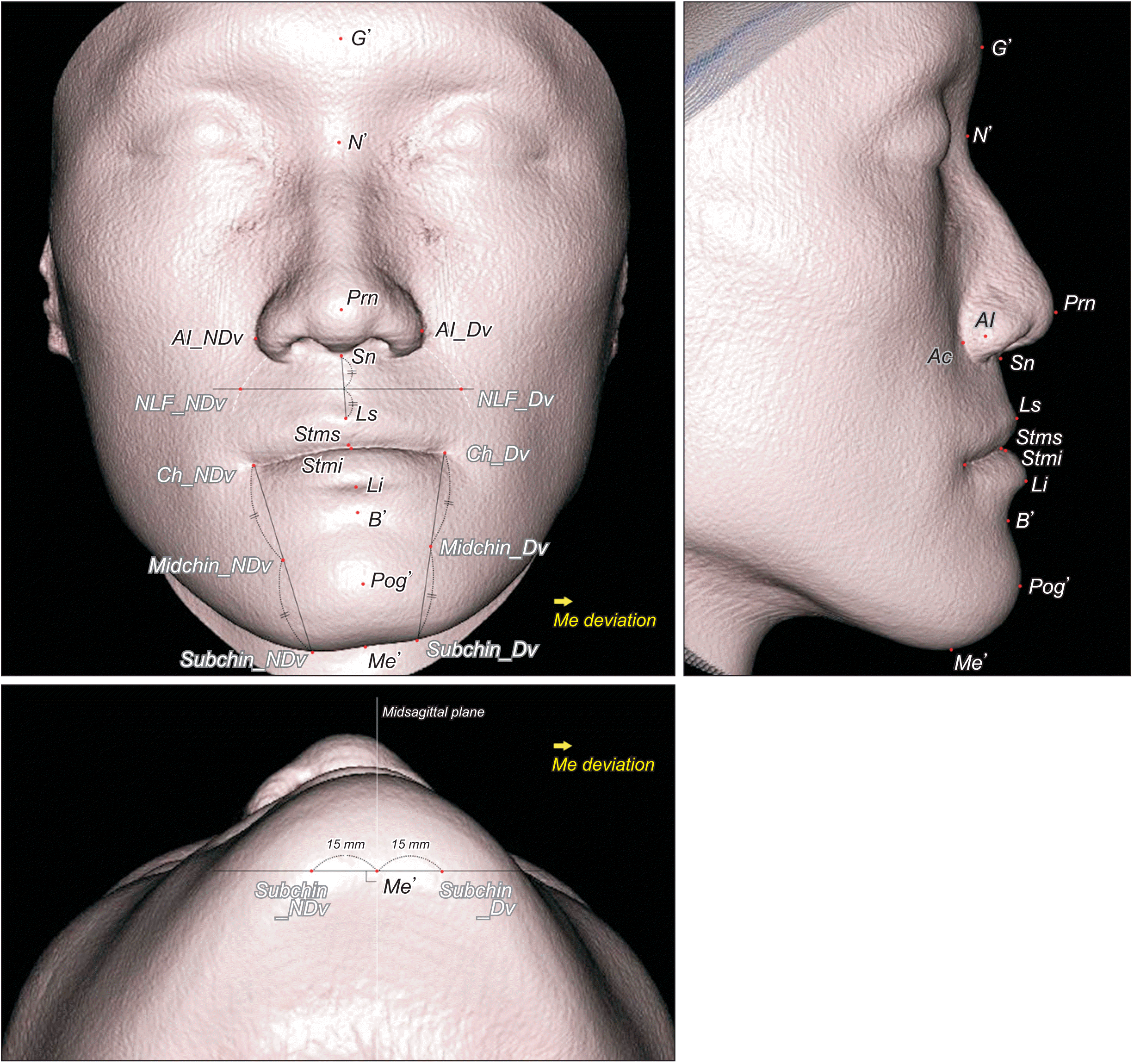 | Figure 3
Soft tissue landmarks investigated in this study.
G’, soft tissue glabella; N’, soft tissue nasion; Prn, pronasale; Al, nasal ala; NDv, nondeviated side; Dv, deviated side; Sn, subnasale; Ls, labrale superius; NLF, nasolabial fold; Stms, stomion superius; Stmi, stomion inferius; Ch, cheilion; Li, labrale inferius; B’, soft tissue B point; Midchin, chin point at the level of midpoint of Ch and Subchin; Subchin, 15 mm lateral to Me’ on the lower chin contour; Pog’, soft tissue pogonion; Me’, soft tissue menton; Ac, nasal alar curvature; Me, menton.

|
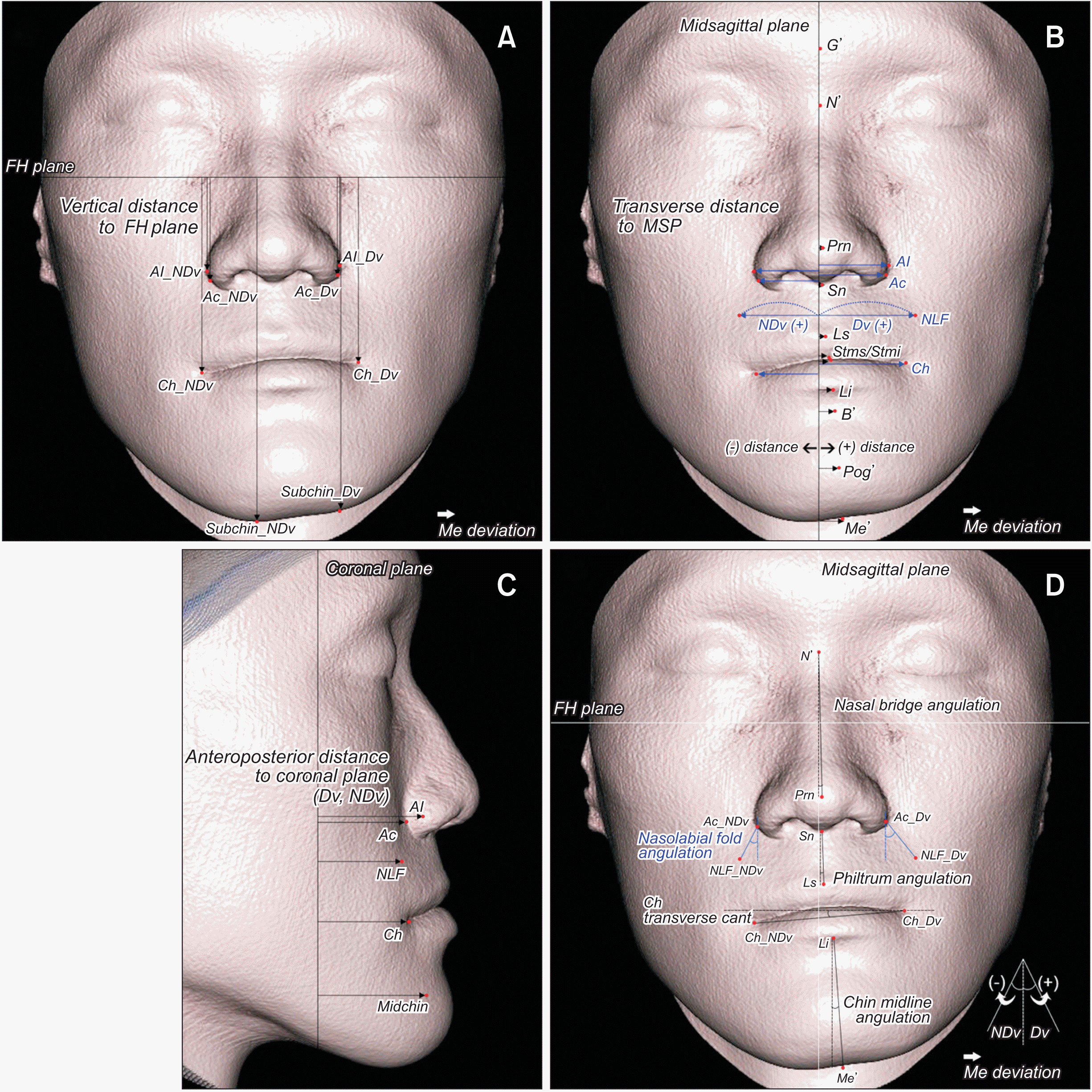 | Figure 4
Soft tissue measurements. A, Vertical distance. B, Transverse distance (blue, bilateral landmarks; black, midline landmarks). C, Anteroposterior distance. D, Line angulation.
FH, Frankfort horizontal; Al, nasal ala; NDv, nondeviated side; Dv, deviated side; Ac, nasal alar curvature; Ch, cheilion; Me, menton; Me’, soft tissue menton; Subchin, 15 mm lateral to Me’ on the lower chin contour; G’, soft tissue glabella; N’, soft tissue nasion; MSP, midsagittal plane; Prn, pronasale; Sn, subnasale; NLF, nasolabial fold; Ls, labrale superius; Stms, stomion superius; Stmi, stomion inferius; Li, labrale inferius; B’, soft tissue B point; Pog’, soft tissue pogonion; Midchin, chin point at the level of midpoint of Ch and Subchin.

|
Table 1
Landmarks and reference planes used in this study
|
Landmark |
Definition |
|
Skeletal |
|
|
Or |
The most inferior point of the lower orbital margin |
|
Po |
The most superior point of the external auditory meatus |
|
Cg |
The most superior point on the crista galli |
|
Op |
The midpoint of the posterior border of the foramen magnum |
|
Go |
The most inferior point of gonial angle on the lateral view |
|
Cd |
The most superior point of the condylar head |
|
Me |
The most inferior point on the symphyseal outline |
|
MF |
The most inferior point of the mental foramen |
|
MFmid |
The midpoint of the MF of both sides |
|
PM |
The point where the curvature changes from concave to convex at the most anterior symphyseal border |
|
Dental |
|
|
UM |
The central fossa of the maxillary first molar |
|
UC |
The cusp tip of the maxillary canine |
|
LM |
The central fossa of the mandibular first molar |
|
LC |
The cusp tip of the mandibular canine |
|
Soft tissue |
|
|
G’ |
The most anterior midpoint of the forehead |
|
N’ |
The most posterior midpoint on the contour of the nasal root |
|
Prn |
The most anterior midpoint of the nasal tip |
|
Sn |
The midpoint located at the junction of the nasal columella and upper lip |
|
Al |
The most lateral point on each nasal alar contour |
|
Ac |
The most posterior point on each alar-facial groove |
|
Ls |
The midpoint of the vermilion line of the upper lip |
|
Li |
The midpoint of the vermilion line of the lower lip |
|
Stms |
The most inferior midpoint of the lower border of the upper lip |
|
Stmi |
The most superior midpoint of the upper border of the lower lip |
|
NLF |
The most posterior point on each nasolabial fold at the vertical level of the midpoint of Sn and Ls |
|
Ch |
The most lateral point on each labial commissure |
|
B’ |
The most posterior midpoint on the mentolabial sulcus |
|
Pog’ |
The most anterior midpoint of the chin |
|
Me’ |
The most inferior midpoint on the lower contour of the chin |
|
Subchin |
The point on each lower contour of the chin at the 15mm lateral to Me’ |
|
Midchin |
The point on each chin contour at the level of the midpoint of Ch and Subchin |
|
Reference plane |
|
|
Frankfort horizontal plane (FHP) |
The plane passing by Po of both sides and right Or |
|
Midsagittal plane (MSP) |
The plane passing by Cg and Op, perpendicular to the FHP |
|
Coronal plane |
The plane passing by Cg, perpendicular to the FHP and MSP |
|
Mandibular horizontal plane (MHP) |
The plane passing by three points, bilateral MFs and PM |
|
Mandibular midsagittal plane (MnMSP) |
The plane passing by Me and MFmid, perpendicular to the MHP |

Table 2
Variables measured in this study
|
Variables |
Definition |
|
Skeletal |
|
|
Distance |
|
|
Menton deviation |
The distance between Me and MSP |
|
Body length |
The distance between Me and Go |
|
Ramus height |
The distance between Go and Cd |
|
Go to MSP |
The distance between the Go and MSP |
|
MFmid deviation |
The distance between the MFmid and MSP |
|
Angulation |
|
|
Ramus inclination |
The angle between the ramus axial line (Cd-Go) and MSP |
|
∠MHP to FHP |
The angle between the MHP and FHP (projected on the coronal plane) |
|
∠MnMSP to MSP |
The angle between the MnMSP and MSP (projected on the FHP) |
|
Dental |
|
|
Vertical distance |
|
|
UM or UC to FHP |
The distance between the UM or UC and FHP |
|
LM or LC to FHP |
The distance between the LM or LC and FHP |
|
Soft tissue |
|
|
Vertical distance |
|
|
Al or Ac to FHP |
The distance between the Al or Ac and FHP |
|
Ch to FHP |
The distance between the Ch and FHP |
|
Subchin to FHP |
The distance between the Subchin and FHP |
|
Transverse distance |
|
|
G’ or N’ to MSP |
The distance between the G’ or N’ and MSP |
|
Prn or Sn to MSP |
The distance between the Prn or Sn and MSP |
|
Al or Ac to MSP |
The distance between the Al or Ac and MSP |
|
NLF to MSP |
The distance between the NLF and MSP |
|
Ls or Li to MSP |
The distance between the Ls or Li and MSP |
|
Stms or Stmi to MSP |
The distance between the Stms or Stmi and MSP |
|
Ch to MSP |
The distance between the Ch and MSP |
|
B’, Pog’, or Me’ to MSP |
The distance between the B’, Pog’, or Me’ to MSP |
|
Anteroposterior distance |
|
|
Al or Ac to coronal plane |
The distance between the Al or Ac and coronal plane |
|
NLF to coronal plane |
The distance between the NLF and coronal plane |
|
Ch to coronal plane |
The distance between the Ch and coronal plane |
|
Midchin to coronal plane |
The distance between the Midchin and coronal plane |
|
Vertical line angulation |
|
|
Nasal bridge to MSP |
The angle between the nasal bridge (N’-Prn) and MSP |
|
Philtrum to MSP |
The angle between the philtrum line (Sn-Ls) and MSP |
|
Chin midline to MSP |
The angle between the chin midline (Li-Me’) and MSP |
|
Nasolabial fold to MSP |
The angle between the nasolabial fold line (Ac-NLF) and MSP |
|
Transverse line angulation |
|
|
Ch line to FHP |
The angle between the Ch line (Ch_NDv-Ch_Dv) and FHP |

For the distance variables of the soft tissue, landmarks were evaluated in the sagittal, vertical, and transverse directions (
Figure 4). To perform a reliable comparison of soft and hard tissues, cranium-based reference planes, such as the FHP and MSP, were equally used to measure the soft tissue, skeletal, and dental variables.
9,23 For bilateral landmarks, the difference in the distance between the deviated (Dv) and nondeviated (NDv) sides (ΔNDv−Dv) was calculated. For midline landmarks, a positive value of the distance was set as the landmark positioned at the Dv, and a negative value was set as the landmark positioned at the NDv.
To further investigate the facial soft tissue, new landmarks of the nasolabial fold and chin area, such as NLF, Midchin, and Subchin, were included in this study. To assess the overall patterns of soft tissue deviation, the angulations of the facial midline and transverse line, such as the nasal bridge, philtrum, chin midline, and transverse lip line, were calculated.
To compare soft tissue measurements with underlying skeletal or dental measurements, cranium-based planes, such as the MSP, FHP, and coronal planes, were used as reference planes (
Figure 1). In addition, to compare the differences in the soft tissue and skeletal chin deviations between the groups, the ratio of soft tissue (Me’) deviation to hard tissue (Me) deviation was calculated for each group. Correlations between soft tissue and skeletal/dental measurements were calculated to determine the relationship between soft and hard tissue deviations.
All the measurements were performed by a single investigator (HJ Kim). For the reliability test, 10 randomly selected patients were reevaluated by the same investigator after 2 weeks. The mean intraclass correlation coefficient was 0.977 (range, 0.957–0.977), and the mean Dahlberg errors were 0.72 mm (range, 0.46–0.86) and 0.71° (range, 0.37–1.07).
The Kolmogorov–Smirnov test showed data normality. A paired t test was used to compare the variables of the sides, and one-way analysis of variance with Tukey’s post-hoc test was performed to compare the variables of the three groups. Linear-by-linear association was used to compare the sex distribution between the groups, and Pearson’s correlation coefficient was calculated to determine the relationship between soft tissue and skeletal/dental variables. Statistical significance was set at P < 0.05, and SPSS statistical software (version 22; IBM Corp., Armonk, NY, USA) was used.
Go to :

RESULTS
Comparison of sex distribution, age, and cephalometric measurements between the groups
No significant differences in sex distribution or age were observed between the three mandibular asymmetry groups (
Table 3). In terms of the sagittal and vertical skeletal patterns, the patients in this study did not show a significant difference in cephalometric measurements between the groups.
Table 3
Demographic characteristics and cephalometric measurements of the sample
|
Roll |
Yaw |
Translation |
P value |
|
Demographic characteristics |
|
|
|
|
|
Sex |
|
|
|
0.787 |
|
Male (n) |
19 |
22 |
18 |
|
|
Female (n) |
11 |
8 |
12 |
|
|
Age (yr) |
21.60 ± 2.33 |
21.59 ± 2.17 |
22.05 ± 3.66 |
|
|
Cephalometric measurements |
|
|
|
|
|
SNA (°) |
80.08 ± 3.04 |
80.66 ± 2.52 |
81.57 ± 3.20 |
|
|
SNB (°) |
82.34 ± 3.08 |
84.07 ± 4.52 |
84.28 ± 3.45 |
|
|
ANB (°) |
−2.26 ± 2.03 |
−3.40 ± 3.20 |
−2.79 ± 2.60 |
|
|
FMA (°) |
27.88 ± 5.03 |
27.19 ± 5.55 |
25.28 ± 5.66 |
|

Comparison of skeletal measurements between the sides and between the groups
Comparison of bilateral measurements between the Dv and NDv revealed that body length and ramus height and inclination were significantly greater at the NDv than those at the Dv (
P < 0.05) in each group (
Table 4). In addition, the distance between the gonion (Go) and MSP was longer at the Dv than at the NDv (
P < 0.001).
Table 4
Comparison of skeletal measurements between the Dv and NDv and between the three mandibular asymmetry groups
|
Roll |
|
Yaw |
|
Translation |
|
Mean ± SD |
P value
(between the sides) |
Mean ± SD |
P value
(between the sides) |
Mean ± SD |
P value
(between the sides) |
|
Me deviation (mm) |
9.28 ± 3.06a
|
- |
|
8.93 ± 3.80a
|
- |
|
6.74 ± 2.13b
|
- |
|
Body length (mm) |
|
|
|
|
|
|
|
|
|
Dv |
80.69 ± 5.26 |
0.002**
|
|
80.33 ± 5.00 |
< 0.001***
|
|
80.65 ± 5.13 |
0.023*
|
|
NDv |
82.06 ± 5.50 |
|
|
84.87 ± 5.44 |
|
|
82.07 ± 5.16 |
|
|
ΔNDv−Dv |
1.37 ± 2.20a
|
- |
|
4.54 ± 2.79b
|
- |
|
1.42 ± 3.25a
|
- |
|
Ramus height (mm) |
|
|
|
|
|
|
|
|
|
Dv |
65.36 ± 6.32 |
< 0.001***
|
|
68.86 ± 6.33 |
< 0.001***
|
|
68.53 ± 7.29 |
< 0.001***
|
|
NDv |
73.44 ± 6.35 |
|
|
71.02 ± 5.80 |
|
|
71.41 ± 6.70 |
|
|
ΔNDv−Dv |
8.09 ± 3.64a
|
- |
|
2.16 ± 2.95b
|
- |
|
2.88 ± 2.43b
|
- |
|
Ramus inclination (°) |
|
|
|
|
|
|
|
|
|
Dv |
1.88 ± 2.36a
|
< 0.001***
|
|
2.47 ± 2.20a,b
|
0.001**
|
|
3.45 ± 2.46b
|
< 0.001***
|
|
NDv |
7.34 ± 2.64a
|
|
|
4.77 ± 2.99b
|
|
|
7.57 ± 3.15a
|
|
|
ΔNDv−Dv |
5.46 ± 2.89a
|
- |
|
2.30 ± 3.41b
|
- |
|
4.11 ± 3.73a,b
|
- |
|
MFmid deviation (mm) |
7.12 ± 2.70 |
- |
|
6.91 ± 3.43 |
- |
|
5.92 ± 2.03 |
- |
|
Go to MSP (mm) |
|
|
|
|
|
|
|
|
|
Dv |
50.88 ± 4.64 |
< 0.001***
|
|
49.35 ± 3.06 |
< 0.001***
|
|
50.04 ± 3.80 |
< 0.001***
|
|
NDv |
43.89 ± 4.11a
|
|
|
46.48 ± 4.23b
|
|
|
43.31 ± 3.60a
|
|
|
ΔNDv−Dv |
−7.00 ± 5.14a
|
- |
|
−2.87 ± 3.67b
|
- |
|
−6.73 ± 4.12a
|
- |
|
MHP to FHP (°) |
6.18 ± 1.24a
|
- |
|
0.87 ± 1.28b
|
- |
|
1.62 ± 0.94c
|
- |
|
MnMSP to MSP (°) |
1.76 ± 1.08a
|
- |
|
8.35 ± 3.41b
|
- |
|
2.06 ± 0.74a
|
- |

Regarding the comparison of the skeletal variables between the groups, the amount of Me deviation was significantly greater in the roll- and yaw-dominant groups (roll, 9.28 mm; yaw, 8.93 mm) than in the translation-dominant group (6.74 mm; P < 0.05). For the bilateral body length difference and angulation between the MnMSP and MSP, the yaw-dominant group presented the highest values (ΔNDv−Dv of body length, 4.54 mm; ∠MnMSP to MSP, 8.35°; P < 0.05) among the three groups. In addition, the bilateral ramus height difference and angulation between the MHP and FHP were significantly higher in the roll-dominant group (ΔNDv−Dv of ramus height, 8.09 mm; ∠MHP to FHP, 6.18°; P < 0.05) than in the other groups. Thus, the roll-dominant group showed higher mandibular rolling asymmetry, and the yaw-dominant group presented higher mandibular yawing asymmetry.
Comparison of dental measurements between the sides and between the groups
As presented in
Table 5, all vertical distances of the canine and first molar of both jaws were significantly greater at the NDv than those at the Dv in the three groups (
P < 0.001), except for the maxillary canine and first molar of the translation-dominant group.
Table 5
Comparison of dental measurements between the Dv and NDv and between the three mandibular asymmetry groups
|
Roll |
|
Yaw |
|
Translation |
|
Mean ± SD |
P value
(between the sides) |
Mean ± SD |
P value
(between the sides) |
Mean ± SD |
P value
(between the sides) |
|
UM to FHP |
|
|
|
|
|
|
|
|
|
Dv |
49.75 ± 3.83 |
< 0.001***
|
|
50.18 ± 3.91 |
< 0.001***
|
|
49.24 ± 3.79 |
0.170 |
|
NDv |
51.70 ± 4.03 |
|
|
51.31 ± 3.99 |
|
|
49.62 ± 3.87 |
|
|
ΔNDv−Dv |
1.94 ± 1.58a
|
- |
|
1.13 ± 1.37a,b
|
- |
|
0.38 ± 1.47b
|
- |
|
UC to FHP |
|
|
|
|
|
|
|
|
|
Dv |
55.03 ± 3.75 |
< 0.001***
|
|
55.29 ± 4.75 |
< 0.001***
|
|
53.64 ± 4.21 |
0.060 |
|
NDv |
56.65 ± 3.96 |
|
|
56.12 ± 4.60 |
|
|
54.12 ± 4.19 |
|
|
ΔNDv−Dv |
1.62 ± 1.18a
|
- |
|
0.83 ± 1.01b
|
- |
|
0.48 ± 1.34b
|
- |
|
LM to FHP |
|
|
|
|
|
|
|
|
|
Dv |
52.22 ± 3.97 |
< 0.001***
|
|
53.20 ± 4.69 |
< 0.001***
|
|
52.34 ± 4.39 |
< 0.001***
|
|
NDv |
54.68 ± 4.34 |
|
|
54.98 ± 4.58 |
|
|
53.41 ± 4.47 |
|
|
ΔNDv−Dv |
2.45 ± 1.15a
|
- |
|
1.78 ± 1.30a,b
|
- |
|
1.07 ± 1.32b
|
- |
|
LC to FHP |
|
|
|
|
|
|
|
|
|
Dv |
54.60 ± 4.94 |
< 0.001***
|
|
54.96 ± 5.24 |
< 0.001***
|
|
53.08 ± 4.47 |
< 0.001***
|
|
NDv |
56.67 ± 5.01 |
|
|
56.29 ± 5.11 |
|
|
54.09 ± 4.70 |
|
|
ΔNDv−Dv |
2.07 ± 1.39a
|
- |
|
1.32 ± 0.95a,b
|
- |
|
1.01 ± 1.28b
|
- |

Bilateral differences in the vertical distance were significantly greater in the roll-dominant group than in the yaw- and/or translation-dominant group (P < 0.05).
Comparison of soft tissue measurements between the sides and between the groups
A comparison of bilateral landmarks between the sides, vertically and transversely, revealed that all variables were significantly different (
P < 0.05) except for the vertical distance of the nasal alae (Al) in the translation-dominant group, showing that they were canted-down at the NDv and deviated toward the Dv (
Table 6,
Figures 5–
7). Anteroposteriorly, in the yaw-dominant group, all landmarks at the NDv were located significantly forward than those at the Dv (
P < 0.001), whereas no significant difference was observed in the Midchin. Conversely, the Midchin at Dv was located significantly forward than NDv in the roll- and translation-dominant groups (
P < 0.001). In addition, the angulation of the nasolabial fold was significantly greater at the Dv than that at the NDv in all groups (
P < 0.001), indicating that the nasolabial fold ran rather horizontally at the Dv and vertically at the NDv.
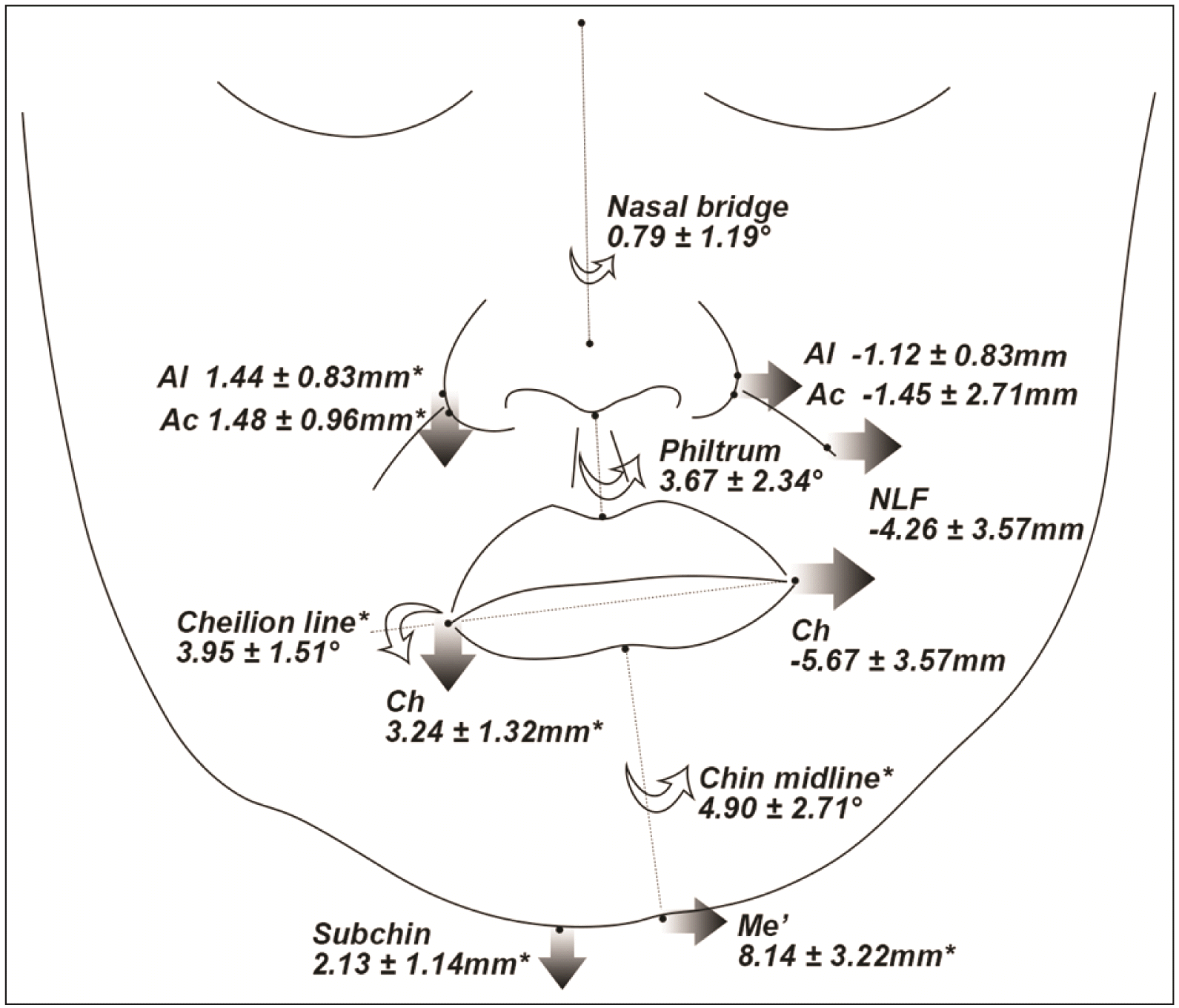 | Figure 5
Schematic illustration of soft tissue deviations or differences in the yaw-dominant group (frontal and modified-submentovertex views; *P < 0.05, significantly greater difference than roll- and/or translation-dominant group).
Al, nasal ala; Ac, nasal alar curvature; Prn, pronasale; Sn, subnasale; NLF, nasolabial fold; Ch, cheilion; Me’, soft tissue menton; Subchin, 15 mm lateral to Me’ on the lower chin contour; Midchin, chin point at the level of midpoint of Ch and Subchin.

|
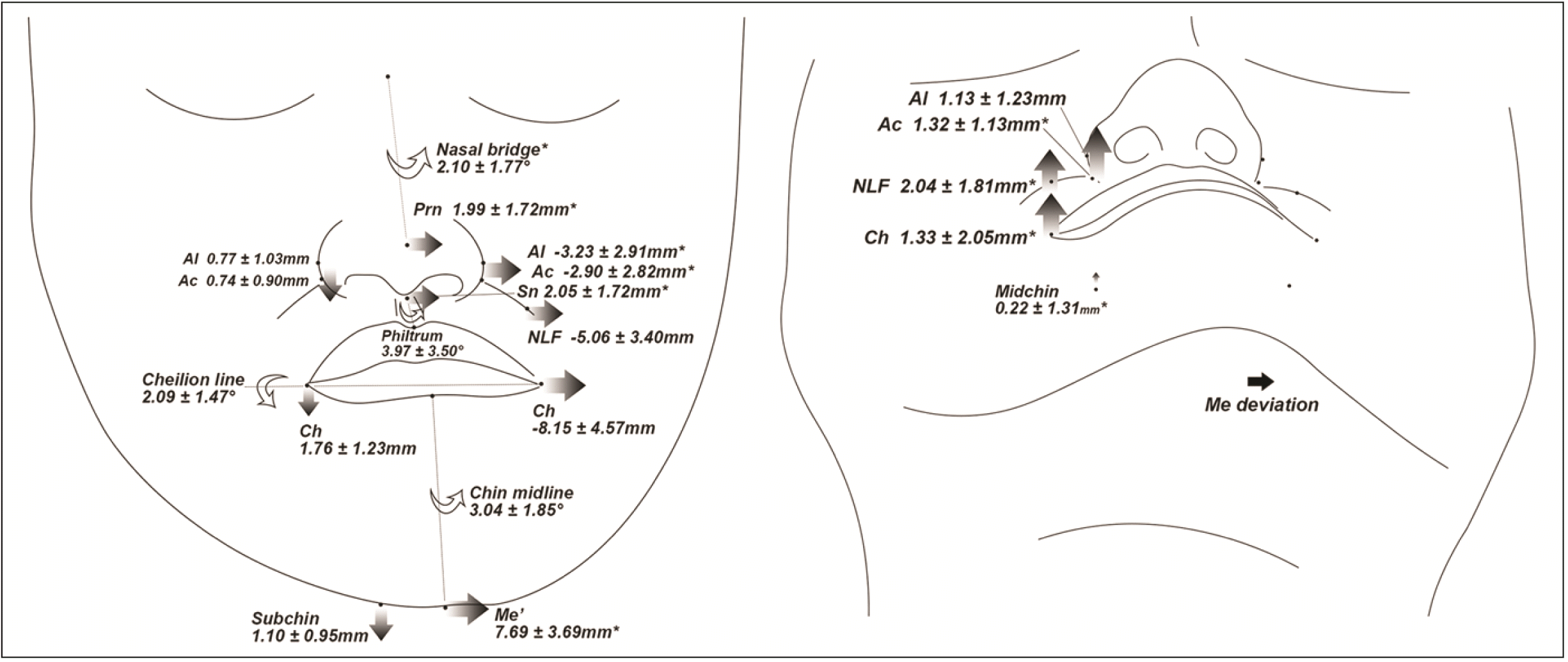 | Figure 6
Schematic illustration of soft tissue deviations or differences in the roll-dominant group (frontal view; *P < 0.05, significantly greater difference than yaw- and/or translation-dominant group).
Al, nasal ala; Ac, nasal alar curvature; NLF, nasolabial fold; Ch, cheilion; Me’, soft tissue menton; Subchin, 15 mm lateral to Me’ on the lower chin contour.

|
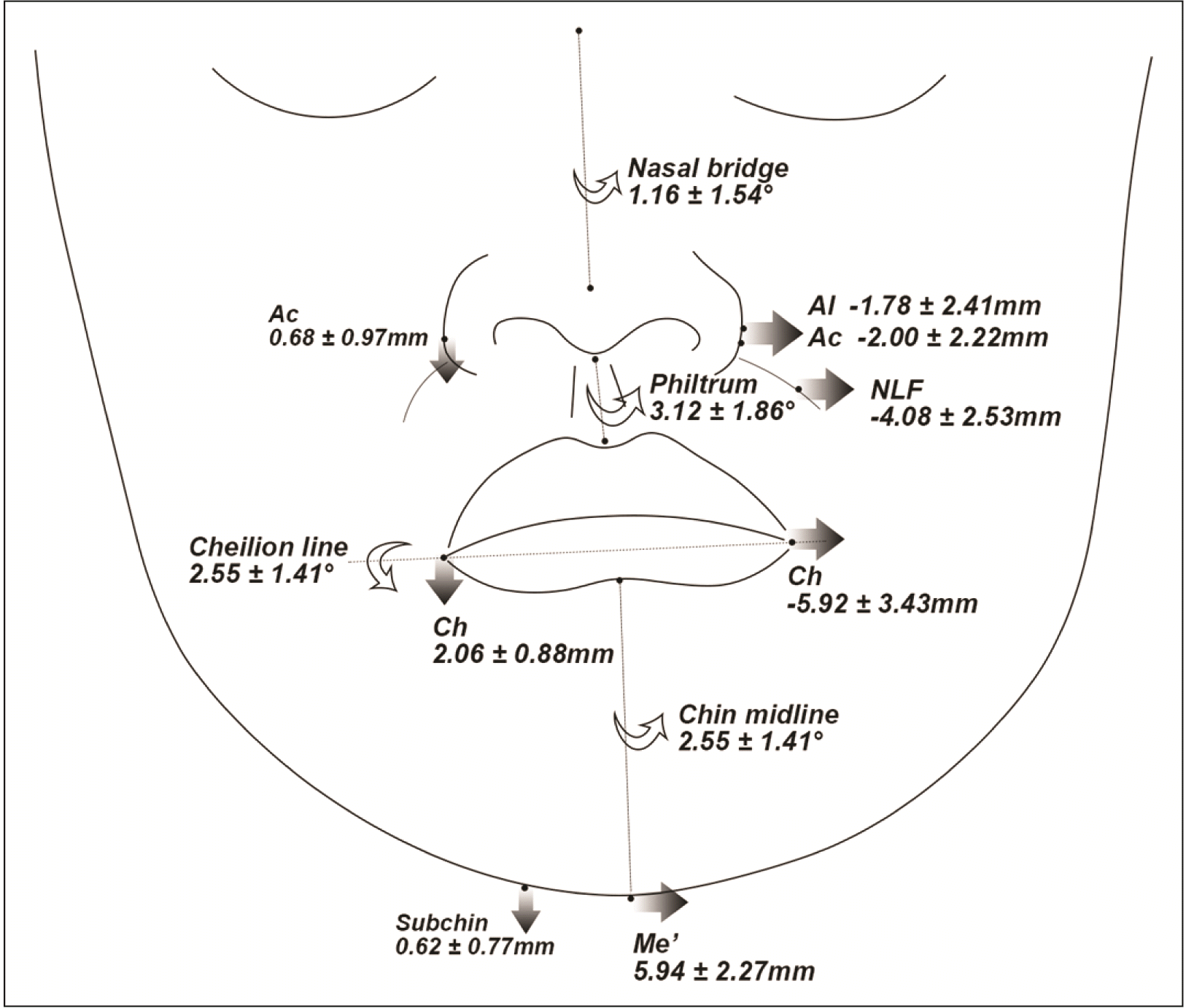 | Figure 7
Schematic illustration of soft tissue deviations or differences in the translation-dominant group (frontal view).
Al, nasal ala; Ac, nasal alar curvature; NLF, nasolabial fold; Ch, cheilion; Me’, soft tissue menton; Subchin, 15 mm lateral to Me’ on the lower chin contour.

|
Table 6
Comparison of soft tissue measurements between the Dv and NDv and between the three mandibular asymmetry groups
|
Roll |
|
Yaw |
|
Translation |
|
Mean ± SD |
P value
(between the sides) |
Mean ± SD |
P value
(between the sides) |
Mean ± SD |
P value
(between the sides) |
|
Vertical distance |
|
|
|
|
|
|
|
|
|
Nasal alae (Al) |
|
|
|
|
|
|
|
|
|
Dv |
24.37 ± 2.85 |
< 0.001***
|
|
24.27 ± 3.00 |
< 0.001***
|
|
23.35 ± 2.67 |
0.181 |
|
NDv |
25.81 ± 2.74 |
|
|
25.04 ± 3.18 |
|
|
24.08 ± 2.55 |
|
|
ΔNDv−Dv |
1.44 ± 0.83a
|
- |
|
0.77 ± 1.03b
|
- |
|
0.73 ± 0.84b
|
- |
|
Nasal alar curvature (Ac) |
|
|
|
|
|
|
|
|
|
Dv |
26.67 ± 2.84 |
< 0.001***
|
|
26.81 ± 3.02 |
< 0.001***
|
|
25.77 ± 2.71 |
0.001**
|
|
NDv |
28.15 ± 2.77 |
|
|
27.55 ± 3.28 |
|
|
26.46 ± 2.49 |
|
|
ΔNDv−Dv |
1.48 ± 0.96a
|
- |
|
0.74 ± 0.90b
|
- |
|
0.68 ± 0.97b
|
- |
|
Cheilion (Ch) |
|
|
|
|
|
|
|
|
|
Dv |
56.41 ± 3.78 |
< 0.001***
|
|
55.94 ± 4.85 |
< 0.001***
|
|
55.14 ± 4.18 |
< 0.001***
|
|
NDv |
59.65 ± 4.19 |
|
|
57.96 ± 5.25 |
|
|
57.21 ± 3.98 |
|
|
ΔNDv−Dv |
3.24 ± 1.32a
|
- |
|
1.76 ± 1.23b
|
- |
|
2.06 ± 0.88b
|
- |
|
Subchin |
|
|
|
|
|
|
|
|
|
Dv |
101.88 ± 6.53 |
< 0.001***
|
|
102.54 ± 6.60 |
< 0.001***
|
|
101.58 ± 8.17 |
< 0.001***
|
|
NDv |
104.02 ± 6.89 |
|
|
103.65 ± 6.97 |
|
|
102.20 ± 8.10 |
|
|
ΔNDv−Dv |
2.13 ± 1.14a
|
- |
|
1.10 ± 0.95b
|
- |
|
0.62 ± 0.77b
|
- |
|
Transverse distance |
|
|
|
|
|
|
|
- |
|
Glabella (G’) |
−0.14 ± 1.06 |
- |
|
0.12 ± 1.16 |
- |
|
−0.20 ± 1.22 |
|
|
Nasion (N’) |
0.06 ± 1.05 |
- |
|
0.27 ± 1.03 |
- |
|
0.11 ± 1.06 |
- |
|
Pronasale (Prn) |
0.69 ± 1.54a
|
- |
|
1.99 ± 1.72b
|
- |
|
0.91 ± 1.48a
|
- |
|
Nasal alae (Al) |
|
|
|
|
|
|
|
|
|
Dv |
19.58 ± 2.02a
|
0.037*
|
|
20.86 ± 2.14b
|
< 0.001***
|
|
19.71 ± 1.78a,b
|
< 0.001***
|
|
NDv |
18.46 ± 1.90 |
|
|
17.63 ± 2.35 |
|
|
17.93 ± 1.53 |
|
|
ΔNDv−Dv |
−1.12 ± 2.81a
|
- |
|
−3.23 ± 2.91b
|
- |
|
−1.78 ± 2.41a,b
|
- |
|
Nasal alar curvature (Ac) |
|
|
|
|
|
|
|
|
|
Dv |
19.87 ± 1.72 |
0.006**
|
|
20.82 ± 1.86 |
< 0.001***
|
|
20.13 ± 1.79 |
< 0.001***
|
|
NDv |
18.41 ± 1.78 |
|
|
17.92 ± 2.48 |
|
|
18.13 ± 1.26 |
|
|
ΔNDv−Dv |
−1.45 ± 2.71 |
- |
|
−2.90 ± 2.82 |
- |
|
−2.00 ± 2.22 |
- |
|
Nasolabial fold (NLF) |
|
|
|
|
|
|
|
|
|
Dv |
30.49 ± 3.40 |
< 0.001***
|
|
31.37 ± 3.58 |
< 0.001***
|
|
29.98 ± 3.48 |
< 0.001***
|
|
NDv |
26.23 ± 3.06 |
|
|
26.31 ± 3.69 |
|
|
25.90 ± 2.90 |
|
|
ΔNDv−Dv |
−4.26 ± 3.57 |
- |
|
−5.06 ± 3.40 |
- |
|
−4.08 ± 2.53 |
- |
|
Subnasale (Sn) |
1.08 ± 1.43a
|
- |
|
2.05 ± 1.72b
|
- |
|
1.30 ± 1.16a,b
|
- |
|
Labrale superioris (Ls) |
1.92 ± 1.54 |
- |
|
2.97 ± 2.15 |
- |
|
1.94 ± 1.37 |
- |
|
Stomion superioris (Stms) |
2.56 ± 1.59 |
- |
|
3.48 ± 2.31 |
- |
|
2.42 ± 1.41 |
- |
|
Stomion inferioris (Stmi) |
3.34 ± 1.69 |
- |
|
4.27 ± 2.56 |
- |
|
3.11 ± 1.71 |
- |
|
Labrale inferioris (Li) |
4.29 ± 1.84 |
- |
|
5.30 ± 2.98 |
- |
|
3.99 ± 1.81 |
- |
|
Cheilion (Ch) |
|
|
|
|
|
|
|
|
|
Dv |
26.08 ± 2.80a
|
< 0.001***
|
|
28.44 ± 3.52b
|
< 0.001***
|
|
26.16 ± 2.59a
|
< 0.001***
|
|
NDv |
20.42 ± 2.62 |
|
|
20.30 ± 3.03 |
|
|
20.24 ± 2.49 |
|
|
ΔNDv−Dv |
−5.67 ± 4.34 |
- |
|
−8.15 ± 4.57 |
- |
|
−5.92 ± 3.43 |
- |
|
Soft tissue B point (B’) |
5.19 ± 2.08 |
- |
|
5.88 ± 3.12 |
- |
|
4.43 ± 1.91 |
- |
|
Soft tissue pogonion (Pog’) |
6.86 ± 2.67a,b
|
- |
|
7.10 ± 3.50a
|
- |
|
5.35 ± 2.13b
|
- |
|
Soft tissue menton (Me’) |
8.14 ± 3.22a
|
- |
|
7.69 ± 3.69a,b
|
- |
|
5.94 ± 2.27b
|
- |
|
Anteroposterior distance |
|
|
|
|
|
|
|
|
|
Nasal alae (Al) |
|
|
|
|
|
|
|
|
|
Dv |
27.55 ± 4.50 |
0.205 |
|
28.07 ± 3.97 |
< 0.001***
|
|
28.40 ± 3.88 |
0.050 |
|
NDv |
27.97 ± 4.64 |
|
|
29.20 ± 4.22 |
|
|
29.01 ± 4.02 |
|
|
ΔNDv−Dv |
0.43 ± 1.81 |
- |
|
1.13 ± 1.23 |
- |
|
0.61 ± 1.63 |
- |
|
Nasal alar curvature (Ac) |
|
|
|
|
|
|
|
|
|
Dv |
22.10 ± 4.51 |
0.278 |
|
22.59 ± 3.34 |
< 0.001***
|
|
23.19 ± 3.76 |
0.113 |
|
NDv |
22.36 ± 4.77 |
|
|
23.91 ± 3.77 |
|
|
23.60 ± 3.91 |
|
|
ΔNDv−Dv |
0.26 ± 1.28a
|
- |
|
1.32 ± 1.13b
|
- |
|
0.41 ± 1.37a
|
- |
|
Nasolabial fold (NLF) |
|
|
|
|
|
|
|
|
|
Dv |
21.39 ± 5.01 |
0.057 |
|
22.53 ± 3.53 |
< 0.001***
|
|
23.19 ± 4.08 |
0.008**
|
|
NDv |
21.84 ± 4.84 |
|
|
24.57 ± 4.36 |
|
|
23.80 ± 4.34 |
|
|
ΔNDv−Dv |
0.46 ± 1.26a
|
- |
|
2.04 ± 1.81b
|
- |
|
0.60 ± 1.16a
|
- |
|
Cheilion (Ch) |
|
|
|
|
|
|
|
|
|
Dv |
24.50 ± 5.45 |
0.120 |
|
26.23 ± 4.67 |
0.001**
|
|
26.97 ± 5.21 |
0.234 |
|
NDv |
23.89 ± 5.12a
|
|
|
27.56 ± 5.12b
|
|
|
26.66 ± 5.08a,b
|
|
|
ΔNDv−Dv |
−0.62 ± 2.10a
|
- |
|
1.33 ± 2.05b
|
- |
|
−0.32 ± 1.43a
|
- |
|
Midchin |
|
|
|
|
|
|
|
|
|
Dv |
25.17 ± 6.12a
|
< 0.001***
|
|
29.73 ± 7.28b
|
0.369 |
|
29.96 ± 6.68b
|
< 0.001***
|
|
NDv |
23.55 ± 6.27a
|
|
|
29.95 ± 7.84b
|
|
|
28.86 ± 6.53b
|
|
|
ΔNDv−Dv |
−1.63 ± 1.67a
|
- |
|
0.22 ± 1.31b
|
- |
|
−1.11 ± 1.21a
|
- |
|
Angulation |
|
|
|
|
|
|
|
|
|
Nasal bridge to MSP |
0.79 ± 1.19a
|
- |
|
2.10 ± 1.77b
|
- |
|
1.16 ± 1.54a
|
- |
|
Philtrum to MSP |
3.67 ± 2.34 |
- |
|
3.97 ± 3.50 |
- |
|
3.12 ± 1.86 |
- |
|
Ch line to FHP |
3.95 ± 1.51a
|
- |
|
2.09 ± 1.47b
|
- |
|
2.55 ± 1.09b
|
- |
|
Chin midline to MSP |
4.90 ± 2.71a
|
- |
|
3.04 ± 1.85b
|
- |
|
2.55 ± 1.41b
|
- |
|
Nasolabial fold to MSP |
|
|
|
|
|
|
|
|
|
Dv |
42.28 ± 7.98 |
< 0.001***
|
|
43.04 ± 7.05 |
< 0.001***
|
|
40.55 ± 7.29 |
< 0.001***
|
|
NDv |
36.75 ± 7.38 |
|
|
38.00 ± 6.91 |
|
|
35.37 ± 8.57 |
|
|
ΔNDv−Dv |
−5.54 ± 5.38 |
- |
|
−5.05 ± 4.36 |
- |
|
−5.17 ± 5.09 |
- |

When comparing ΔNDv−Dv of the vertical distance between the groups, the roll-dominant group exhibited significantly greater values than the other groups (P < 0.05), indicating a bigger transverse line cant of the bilateral landmarks. For the ΔNDv−Dv of transverse distance, the yaw-dominant group showed the greatest difference between the sides, although statistical significance was found only at the Al (P = 0.01). Anteroposteriorly, all ΔNDv−Dv values were significantly greater in the yaw-dominant group than in the other groups (P < 0.05), except that of the Al.
Regarding the transverse distance of midline landmarks, the pronasale (Prn) and subnasale of the yaw-dominant group significantly deviated to the Dv more than those of the roll- and translation-dominant groups (P < 0.05).
Regarding the line angulations, the Nasal bridge (N’-Prn) was significantly inclined to the Dv in the yaw-dominant group (2.10°; P < 0.05) compared with that in the other groups (roll, 0.79°; translation, 1.16°). The angulation of the Chin midline and Ch line was significantly greater in the roll-dominant group (Chin midline, 4.90°; Ch line, 3.95°; P < 0.05) than in the yaw- (Chin midline, 3.04°; Ch line, 2.09°) and translation-dominant groups (Chin midline, 2.55°; Ch line, 2.55°). Philtrum angulation was not significantly different between the groups.
Relationship between the soft tissue and skeletal/dental measurements
The ratio of Me’ deviation to the Me deviation was not significantly different between the groups (roll, 0.87; yaw, 0.84; translation, 0.87) (
Table 7).
Table 7
Comparison of the Me’ deviation ratio relative to Me deviation between the three mandibular asymmetry groups
|
Ratio |
Roll |
Yaw |
Translation |
|
Me’ deviation/Me deviation |
0.87 ± 0.14 |
0.84 ± 0.17 |
0.87 ± 0.13 |

Table 8 shows the correlation between soft tissue line angulation and skeletal/dental measurements. In the roll-dominant group, the angulation of the Chin midline or Ch line was positively correlated with mandibular rolling (∠MHP to FHP;
P < 0.01) and Me and MFmid deviations (
P < 0.001). In addition, the bilateral difference in the vertical position of the mandibular canine or molar showed a positive correlation with the angulation of the Chin midline (mandibular canine,
P < 0.01; mandibular molar,
P < 0.05) or Ch line (mandibular canine,
P < 0.001; mandibular molar,
P < 0.01). In the yaw-dominant group, all soft tissue line angulation variables were positively correlated with Me (Nasal bridge, Philtrum,
P < 0.001; Chin midline, Ch line,
P < 0.01) and MFmid deviations (Nasal bridge, Chin midline,
P < 0.01; Philtrum, Ch line,
P < 0.001). Mandibular yawing (∠MnMSP to MSP) was also positively correlated with the Nasal bridge (
P < 0.01) and Philtrum angulations (
P < 0.001). In the translation-dominant group, the Nasal bridge and Chin midline angulations were positively correlated with Me and MFmid deviations (
P < 0.05). Chin midline or Ch line angulation was positively correlated with bilateral differences in the vertical position of the mandibular canine and molar (
P < 0.05).
Table 8
Pearson’s correlation coefficients between line angulation of soft tissue and skeletal/dental measurements
|
Roll |
|
Yaw |
|
Translation |
|
Nasal bridge |
Philtrum |
Chin midline |
Ch line |
Nasal bridge |
Philtrum |
Chin midline |
Ch line |
Nasal bridge |
Philtrum |
Chin midline |
Ch line |
|
Skeletal |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me deviation |
−0.006 |
0.246 |
0.791***
|
0.728***
|
|
0.574***
|
0.727***
|
0.560**
|
0.547**
|
|
0.405*
|
0.321 |
0.603***
|
0.354 |
|
MFmid deviation |
0.011 |
0.273 |
0.751***
|
0.709***
|
|
0.599**
|
0.696***
|
0.582**
|
0.612***
|
|
0.426*
|
0.289 |
0.568**
|
0.320 |
|
∠MHP to FHP |
−0.068 |
0.241 |
0.554**
|
0.655***
|
|
−0.201 |
0.143 |
0.318 |
0.290 |
|
−0.136 |
0.072 |
0.282 |
0.284 |
|
∠MnMSP to MSP |
−0.001 |
0.242 |
0.239 |
0.325 |
|
0.525**
|
0.684***
|
0.226 |
0.013 |
|
0.200 |
0.113 |
0.145 |
0.177 |
|
Dental (ΔNDv–Dv) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
UM to FHP |
0.238 |
−0.074 |
0.015 |
0.226 |
|
0.168 |
−0.242 |
0.230 |
0.290 |
|
0.257 |
−0.093 |
0.099 |
0.031 |
|
UC to FHP |
0.068 |
0.281 |
−0.087 |
0.115 |
|
0.195 |
−0.064 |
< 0.001 |
0.160 |
|
0.491**
|
−0.052 |
0.092 |
0.166 |
|
LM to FHP |
0.124 |
−0.040 |
0.431*
|
0.521**
|
|
0.309 |
−0.128 |
0.136 |
0.390*
|
|
0.355 |
0.184 |
0.449*
|
0.412*
|
|
LC to FHP |
−0.035 |
0.145 |
0.527**
|
0.632***
|
|
0.121 |
0.075 |
0.291 |
0.365 |
|
0.283 |
−0.113 |
0.410*
|
0.381*
|

Go to :

DISCUSSION
To define the distinct manifestations of facial soft tissues based on each mandibular asymmetry type, the soft tissue variables were compared between the different asymmetry types. In accordance with earlier studies that mentioned that internal skeletal asymmetry was rather masked by the soft tissue,
9,24 the Me’/Me deviation ratio was 0.84 to 0.87 in this study. Interestingly, the ratio was not significantly different between the asymmetry types, indicating that the internal Me deviation was masked by the soft tissue at a uniform rate, irrespective of the roll-, yaw-, or translation-dominant mandibular deviation. The other soft tissue positions assessed in three dimensions, however, were quite different according to the mandibular asymmetry type.
The roll-dominant group demonstrated greater vertical differences in the bilateral landmarks of the soft tissue than the other groups (
Figures 5 and
8). Notably, the transverse lip cant was more prominent than the other groups, and the value was highly related to Me deviation as suggested in previous studies.
10,19 Interestingly, in this study, the lip cant also presented a high correlation with mandibular rolling. This might be related to the depressor anguli oris muscle that is highly associated with the vertical position of the lip corner and is attached to the side of the mandibular body.
25 Once the mandible rolled, the muscle might be positioned more inferior at the NDv than at the Dv, which possibly canted down the lip corner at the NDv. Hence, to correct lip line canting, the Me should be surgically moved toward the NDv sufficiently to position itself on the MSP along with sufficient mandibular rolling correction. Importantly, the transverse occlusal cant of both jaws should be completely corrected in advance to accomplish sufficient mandibular rolling. Regarding soft tissue asymmetry of the chin, the vertical position of the Subchin at the NDv was more inferior than that of the Dv, indicating that the lower chin contour of the NDv was canted down, and the angulation of the Chin midline was positively correlated with mandibular rolling. To achieve bilaterally balanced soft tissues in the chin of the roll-dominant asymmetric mandible, the mandibular rolling correction along with the lateral movement to the NDv could be emphasized repeatedly. If a certain amount of asymmetry remains after mandibular surgery even with proper rolling and positional correction, supplementary osteotomy on the inferior border of the body can be considered.
26,27 Accordingly, in patients with facial asymmetry presenting with moderate-to-severe lip cant and side-to-side discrepancy of the soft tissue around the nose and chin in the vertical direction, treatment would need to focus on mandibular rolling to deal with these soft tissue deviations.
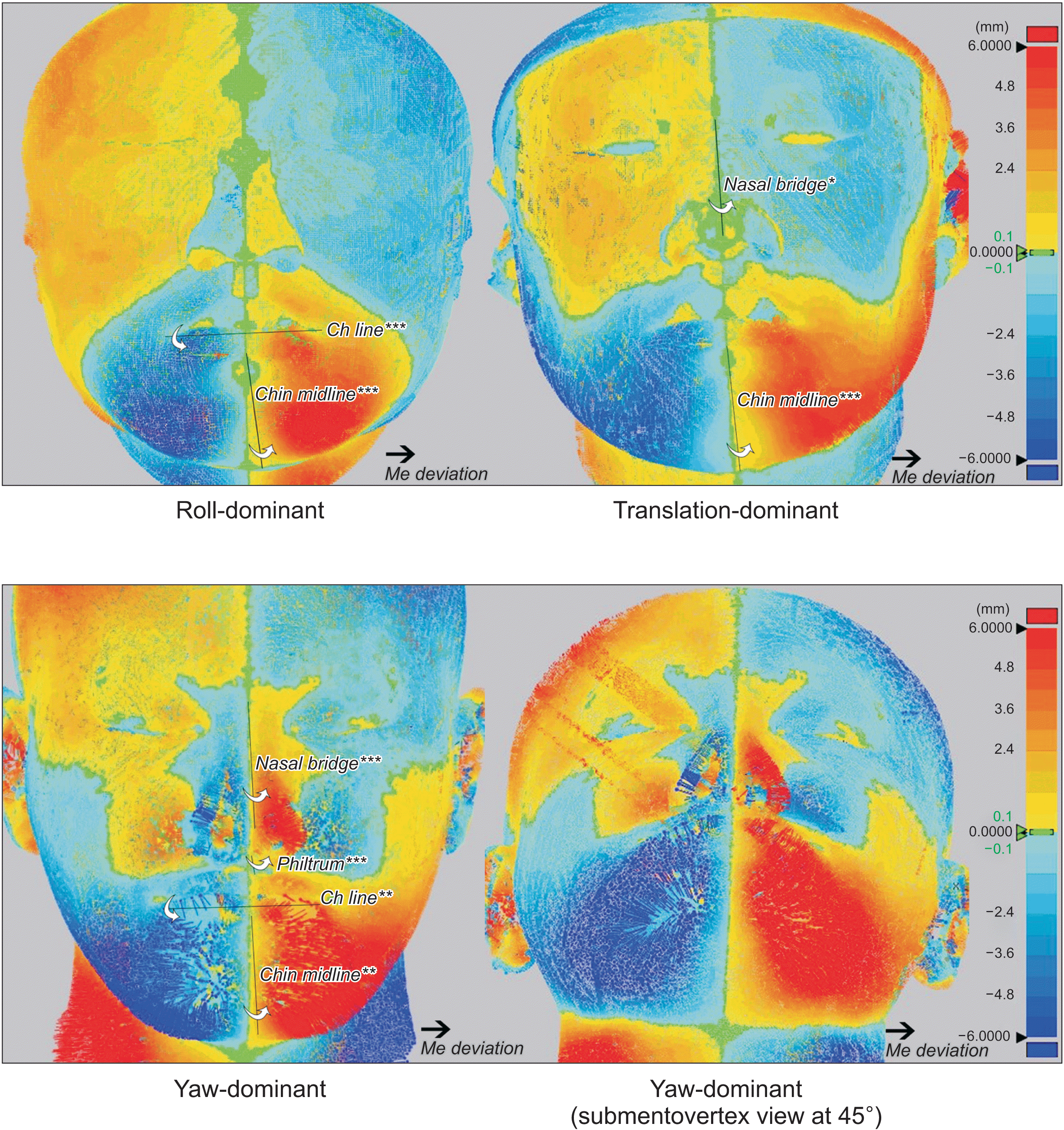 | Figure 8
Color maps showing the soft tissue differences in distance between the original and mirrored images based on the midsagittal plane for each mandibular asymmetry type. The color maps were constructed using three-dimensional analysis software (Geomagic Control X; 3D Systems, Rock Hill, SC, USA). When original soft tissues were positioned out of or lateral to the mirrored images, yellow to red colors were mapped based on the distance, and pale blue to blue colors were mapped for the mirrored images relative to the original images. Line angulations that presented a significant correlation with menton (Me) deviation (*P < 0.05, **P < 0.01, ***P < 0.001).
Ch, cheilion.

|
Meanwhile, for bilateral soft tissue comparison in the yaw-dominant group, the vertical difference was lower than that in the roll-dominant group. However, the lateral deviation of the nose and lips was the greatest among the three asymmetry groups (
Figures 6 and
8). In addition, the extent of mandibular yawing positively correlated with the angulations of the Nasal bridge and Philtrum. This may be attributed to the horizontally-deviated anterior body of the yaw-dominant asymmetric mandible. Previous studies revealed that a deviated nasal septum was more closely associated with the horizontal growth difference than with the vertical difference.
28,29 In addition, some previous studies highlighted that nasal asymmetry correction is more important for facial esthetics than chin asymmetry.
30,31 Therefore, nasal deviation should be detected with attention, particularly in patients with yaw-dominant mandibular asymmetry. If required, rhinoplasty needs to be considered during or after jaw surgery to enhance the symmetry of the face.
32,33 For the anteroposterior positions, the nasal- and lip-related landmarks at the NDv are located more anteriorly than those at the Dv. This bilateral discrepancy in facial frontal projection might be due to the mandibular yaw rotation that can lead to anteroposterior positional differences between the sides.
15 Therefore, to improve this asymmetry, mandibular yaw correction, including more setback movement at the NDv and less setback or advancement at the Dv, would be required during skeletal Class III jaw surgery. Particularly, this frontal projection difference at the nasal-related soft tissue landmarks may be related to nasal deviation;
27 thus, subalar grafting on the depressed Dv can be considered after jaw surgery for better symmetry of the facial projection and nasal axis.
12,34 In clinics, facial photographs of the modified submentovertex view at 45° may also be useful for evaluating the bilateral differences in the soft tissue projection (
Figures 6 and
8). Therefore, if significant lateral deviations of the nasal- and lip-related soft tissues and bilateral differences in facial projection are observed, sufficient yaw correction of the mandible might be required in treatment planning. Thereafter, supplementary graft surgery would be considered if needed. Transverse lip cant in this group was highly correlated with Me and body deviation to the MSP, but not with mandibular yawing; thus, sufficient lateral body movement to the NDv would be required for a balanced lip line.
Patients with translation-dominant mandibular asymmetry showed differences in the vertical and transverse directions between the bilateral landmarks (
Figures 7 and
8). Despite the lower Me or Me’ deviation, the translation-dominant group presented a similar or not significantly lesser transverse deviation of landmarks around the lips (Ls, Li, Stms, Stmi, and B’) compared with the other groups. Hence, lateral translation of the mandible to the Dv, might contribute to lip deviation. To fully improve the deviated soft tissues, the mandible should be moved to the NDv by bodily translation along with the lateral movement of the Go during jaw surgery.
Collectively, facial soft tissues showed different deviation patterns according to the mandibular asymmetry types. Even though the amount of hard-tissue menton deviations did not differ according to the types in this study, the facial soft tissues were quite different. Therefore, the orthognathic surgical plan, including orthodontic decompensation, should differ according to type. The roll-dominant group was closely associated with the vertical difference between the sides, and the amount of mandibular rolling presented a positive correlation with transverse lip cant. Thus, proper correction of mandibular rolling is imperative in jaw surgery accompanied by appropriate dental decompensation. By contrast, the yaw-dominant group demonstrated a large extent of soft tissue deviation, both transversely and anteroposteriorly. In particular, the extent of mandibular yawing was highly correlated with nasal and philtrum asymmetries. Therefore, sufficient lateral movement of the mandible with yaw correction is mandatory, and rhinoplasty should be considered in treatment planning. From a clinical viewpoint, the surgical plan needs to differ according to mandibular asymmetry type, although the soft tissue menton deviation is the same. The relationship between soft and hard tissue deviations evaluated in this study may be useful in establishing guidelines for appropriate tooth and jaw movements to accomplish satisfactory facial esthetics.
Although this study successfully assessed soft tissue deviations in each mandibular asymmetry type, the soft tissue changes related to hard tissue movements by treatment were not evaluated. Therefore, further investigation is needed to determine the differences in soft tissue changes after treatment between different asymmetry types.
Go to :













 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download