Abstract
Enamel demineralization represents the most prevalent complication arising from fixed orthodontic treatment. Its main etiology is the development of cariogenic biofilms formed around orthodontic appliances. Ordinarily, oral biofilms exist in a dynamic equilibrium with the host's defense mechanisms. However, the equilibrium can be disrupted by environmental changes, such as the introduction of a fixed orthodontic appliance, resulting in a shift in the biofilm’s microbial composition from non-pathogenic to pathogenic. This alteration leads to an increased prevalence of cariogenic bacteria, notably mutans streptococci, within the biofilm. This article examines the relationships between oral biofilms and orthodontic appliances, with a particular focus on strategies for effectively managing oral biofilms to mitigate enamel demineralization around orthodontic appliances.
Orthodontic treatment, while mostly successful, can come with various side effects related to the use of orthodontic appliances. One of the most common side effects is enamel demineralization, characterized by the formation of white spot lesions around fixed orthodontic appliances.1-3 These appliances promote the accumulation of biofilms by offering additional binding sites for oral bacteria and impeding oral hygiene practices. Consequently, this leads to disruptions in oral microbial homeostasis, including enhanced biofilm formation and an increased presence of cariogenic bacteria, particularly mutans streptococci (MS), within these biofilms. Such qualitative and quantitative changes in microbial composition alter the interaction between bacteria and the host's defense mechanisms, resulting in white spot lesions through decreased enamel mineral content, alterations in inorganic chemical composition, and consequent changes in optical properties.
Enamel demineralization associated with orthodontic treatment primarily manifests on the maxillary incisors, with reported prevalence rates of up to 50%.2,3 However, these rates vary depending on patient cooperation, detection methods, inclusion criteria, and preventive measures. Notably, these lesions can compromise the esthetic outcomes of orthodontic treatment and, more concerningly, may be potentially irreversible, persisting for up to 12 years after the removal of orthodontic appliances.4 Therefore, the primary emphasis should be on prevention rather than post-occurrence management of white spot lesions.
Microbiological research concerning fixed orthodontic appliances has been a prominent area of interest in orthodontics in recent decades, focusing on identifying and preventing enamel demineralization. Orthodontic research primarily targets 2 cariogenic MS strains, Streptococcus mutans (S. mutans) and Streptococcus sobrinus (S. sobrinus), as they are the most frequently isolated species in the human oral cavity.5,6 In the early stages of orthodontic microbial research, most studies centered on the adhesion of single bacterial species, namely S. mutans or S. sobrinus, to various orthodontic appliances.7-14 For quantitative analyses, MS adhesion to appliance surfaces was assessed using methods like direct counting (via optical and electron microscopes) and indirect techniques (optical absorbance and colony counting). However, since the oral microbiota is diverse and comprises various microbial species, studies involving only 1 or 2 bacterial strains have limitations in replicating the complexity of the oral environment. Furthermore, classical culture-based methods were time-consuming, labor-intensive, and relatively imprecise, contributing to inconsistent findings in orthodontic microbial research.
As science and technology have advanced, molecular biological approaches incorporating specific genetic analyses, such as real-time polymerase chain reaction, have emerged in microbial research within orthodontics.15-19 These gene-based methods offer higher sensitivity and specificity in detecting and quantifying multiple pathogenic bacteria associated with orthodontic appliances, enabling more accessible, rapid, and accurate analyses.
Although many studies have shown that wearing a fixed orthodontic appliance can influence the levels of periodontopathic microorganisms in biofilms,17,18,20,21 this review primarily focuses on alterations in biofilms surrounding orthodontic appliances, specifically changes in the composition of cariogenic microorganisms within these biofilms linked to enamel demineralization. This article aims to explore how orthodontic procedures and appliances can impact biofilm formation associated with enamel demineralization, particularly in terms of changes in the composition of cariogenic microorganisms within biofilms formed around orthodontic appliances.
The formation of biofilms around fixed orthodontic appliances significantly impacts enamel demineralization. Therefore, comprehending the biofilm formation process is crucial to preventing enamel demineralization.
The process of biofilm formation begins with the attachment of salivary pellicles to intraoral surfaces. Immediately after cleaning and polishing, oral surfaces become coated with saliva. This salivary pellicle also forms on orthodontic appliances, and the interactions between salivary components in the pellicles and microorganisms play a pivotal role in the initial microbial adherence, which is the primary step of biofilm formation around orthodontic appliances (Figure 1).22,23 The composition of salivary pellicles varies depending on the physicochemical characteristics of the orthodontic appliances, and the number and type of bacteria initially attached to intraoral surfaces are influenced by the receptors on the salivary pellicles.22,24
Generally, salivary glycoproteins like agglutinin and mucin within the pellicles act as receptors for the adhesion of initial colonizers, such as oral streptococci, to orthodontic materials.25 Following the adhesion of these initial colonizers, filamentous and gram-negative species become part of the biofilms, which are then followed by divergence flora containing motile rods and spirochetes (Figure 1).26,27 The progressive development of biofilms is associated with physiological changes within them. Bacteria that can thrive in an aerobic environment are initially involved in the initial biofilm formation, and as the biofilm matures, it transitions into an anaerobic environment, creating favorable conditions for gram-negative anaerobic species to thrive.28 Consequently, increased levels of anaerobic periodontal pathogens in biofilms, particularly in subgingival areas, disrupt the host’s defense mechanisms, resulting in gingival inflammation and/or attachment loss during orthodontic treatment with a fixed appliance. However, the proportion of anaerobic bacteria in biofilms around interproximal and/or occlusal areas may not significantly increase beyond a certain limit as oxygen is supplied. Instead, the proportion of facultative bacteria, such as MS, may increase over time.17-19
While biofilms develop through complex interactions among various strains, most orthodontic microbial studies investigating bacterial interactions with orthodontic appliances have used single species, predominantly S. mutans.7-12,24 Moreover, saliva has been rarely included in orthodontic microbial research. Therefore, there have been limitations in simulating the actual oral environment where saliva and bacteria interact. To address these limitations, multispecies biofilm research using saliva has been initiated in orthodontics and is gradually expanding to in vivo and in situ studies.15-18,29 These studies hold great potential for preventing enamel demineralization induced by biofilms formed around orthodontic appliances. This review will focus on the latest microbial research in the field of orthodontics.
Using fixed orthodontic appliances introduces a distinctive environmental change, notably an elevation in the proportion of MS within the biofilms surrounding these appliances. Moreover, it has been observed that MS levels tend to persistently increase even after the removal of orthodontic appliances.15 This escalating presence of MS in biofilms serves as the primary catalyst for enamel demineralization and the development of dental caries in orthodontic patients. Thus, it is imperative to comprehend the distinctive characteristics of MS concerning biofilm formation and its implications for enamel demineralization.
Mutans streptococci are the members of the initial colonizers that contribute to biofilm formation using their ability to adhere to the salivary pellicles formed on tooth surfaces.25,28 In addition, they produce lactic acid through glycolysis, inducing enamel demineralization and dental caries around orthodontic appliances.
The main cariogenic property of MS originates from glucans, sticky extracellular polysaccharides synthesized from sucrose by glucosyltransferases on the surface of MS.27,28,30,31 The glucans serve as extracellular matrices for oral biofilms, facilitating the attachment and colonization of oral bacteria during biofilm maturation (Figures 2 and 3).25,28 In addition, the glucan matrix inhibits the diffusion of acids produced within the biofilm into the oral cavity, thereby prolonging the contact of acids with the tooth surface.26 Consequently, the concentration of acids increases, subsequently lowering the pH within the biofilm and resulting in enamel demineralization. Furthermore, the acidic microenvironment within the biofilm interferes with the growth of commensal bacteria while fostering a favorable milieu for the proliferation of acid-resistant MS.26,28 Moreover, the relatively anaerobic environment with biofilm maturation alters the cariogenic properties of S. mutans, amplifying its capacity to develop biofilms, generate acids, and thrive within the acidic surroundings.32 Hence, as biofilms mature with an escalation in glucan production, the internal environment of these biofilms gradually becomes cariogenic, consequently shifting the biofilm equilibrium and increasing the proportion of MS within the biofilms. Multispecies biofilm studies have consistently indicated an increase in MS within biofilms formed around orthodontic appliances over time.17,18 All these findings underscore the urgency of promptly removing biofilms surrounding orthodontic appliances before they reach full maturation.
Research focused on oral biofilms has underscored the pivotal role played by glucans in bacterial adhesion and biofilm formation. Therefore, the primary strategy for averting enamel demineralization involves the inhibition of glucan production and accumulation. Notably, there exists a significant association between sugar intake and increased dental caries rates.30,31 This can be achieved by reducing the consumption of sugar-containing foods and maintaining rigorous oral hygiene, particularly for orthodontic patients who struggle with oral hygiene maintenance.
The successful attachment of orthodontic appliances to tooth surfaces hinges on orthodontic bonding procedures. These procedures, encompassing acid etching, priming, and the application of bonding adhesives to tooth surfaces, substantially impact bacterial adhesion and biofilm formation due to their capacity to alter the surface characteristics of teeth.
Several studies have sought to pinpoint which step in the bonding process has the most pronounced effect on bacterial adhesion and biofilm formation.13,18,33 Notably, studies involving MS and hydroxyapatite have shown that acid etching leads to a significant increase in MS adhesion to etched surfaces compared to untreated, primed, or adhesive surfaces (Figure 3).13,33 This phenomenon arises from the heightened surface roughness and surface-free energy achieved through acid etching (Figure 4A and 4B).11,13,18 Of particular concern is that acid etching significantly promotes biofilm formation and increases the proportion of MS within these biofilms.13,18 Given that enamel demineralization tends to manifest within 4 weeks of bonding orthodontic appliances,34 these findings suggest that, in terms of biofilm formation and MS colonization, minimizing the extent of unnecessary etched surfaces can aid in the prevention of enamel demineralization, even if the etched surface undergoes remineralization over time following interactions with saliva.35
However, the question arises: How should the etched surface be managed to prevent enamel demineralization? To address this, it is essential to consider that biofilm formation tends to be reduced on primed or adhesive surfaces compared to etched surfaces.13,18,33 Consequently, any residual etched surface following orthodontic bonding should be coated with orthodontic primers or adhesives to curtail biofilm formation. Yet, applying primers and adhesives to tooth surfaces is not without its challenges.
Orthodontic primers and adhesives are primarily resin-based materials, comprising various monomers such as bisphenol A glycidyl methacrylate (bis-GMA) and urethane dimethacrylate (UDMA), along with other components including initiators, activators, and inhibitors. Following polymerization, unreacted elements are released into the oral cavity, particularly in the immediate aftermath of this process.36 Furthermore, these materials continuously release leachable elements into the oral environment due to mechanical erosion, pH fluctuations, thermal cycling, and hydrolysis by salivary enzymes.37 Moreover, acidic biofilms can degrade and roughen the surfaces of these materials, which, in turn, facilitate the release of leachable elements.38 These leachable elements can significantly influence bacterial adhesion and biofilm formation in the vicinity of orthodontic appliances, particularly at the interface between enamel and resin-based orthodontic materials.
Several studies have been conducted to investigate the effects of the leachable elements on bacterial adhesion and biofilm formation of S. mutans.39,40 Among them, bis-GMA and UDMA, the cross-linking monomers that help to decrease the conversion rate, promote biofilm formation in S. mutans by increasing bacterial adhesion, sugar transport, and glucan synthesis (Figure 5).39,40 These indicate that leachable monomers from orthodontic primers and/or adhesives may induce biofilm-related enamel demineralization at the junction between tooth surfaces and resin-based orthodontic materials by changing the virulence of S. mutans. Effects of residual monomers on bacterial adhesion and biofilm formation can also partly explain why biofilm and/or calculus deposition occurs well on the surfaces of the removable orthodontic appliances and the gingival margin of the fixed retainers in clinical situations. Since hydrophobic bis-GMA and UDMA are not washed out well by saliva, they can remain at the interface between the materials and enamel for a long time. To reduce biofilm formation and MS colonization, orthodontic primers and adhesives should be applied as thinly as possible, and the residual monomers around the resin-based orthodontic materials should be mechanically removed using periodic oral prophylaxis. Considering that cross-linking monomers, such as bis-GMA, are a major component that accounts for about 50% of orthodontic primers, unbound monomers are mostly present during the first 24 hours after polymerization,36 and they can significantly promote glucan synthesis and biofilm formation by reacting with sucrose,39,40 it is recommended to ask patients to avoid sugar-containing foods during at least 24 hours after orthodontic bonding.
To prevent biofilm formation and enamel demineralization around the primed enamel surface by leachable monomers, there was an attempt to incorporate antimicrobial agents into orthodontic primers. Chlorhexidine (CHX), the clinically proven antimicrobial substance in dentistry, was successfully incorporated into an orthodontic primer, significantly inhibiting the biofilm formation of MS on its surfaces without significantly influencing bond strength or bond failure patterns.41 In addition to CHX, orthodontic primers containing various antimicrobial substances have been introduced and examined.42,43 These attempts may help to prevent enamel demineralization associated with orthodontic bonding procedures.
The research results on the relationships between residual monomers and cariogenic bacteria indicate the need for extensive research on leachable elements that affect enamel demineralization associated with orthodontic materials, and the effects of orthodontic materials on biofilm formation should be evaluated as a principal factor during the process of determining the biocompatibility.
Orthodontic brackets are integral components of fixed orthodontic appliances, playing a substantial role in the development of enamel demineralization during long-term orthodontic treatment. Their intricate design poses challenges for maintaining oral hygiene, hindering effective cleaning around orthodontic appliances, and creating additional surfaces for biofilm formation upon attachment to tooth surfaces. Consequently, the adhesion and biofilm formation of cariogenic bacteria associated with orthodontic brackets have been a focal point in orthodontic research. However, previous studies have yielded diverse results due to variations in bracket size, shape, and non-standardized research methodologies utilizing only 1 or 2 bacterial species.7,9,44,45 This lack of standardization has contributed to inconclusive findings regarding which type of bracket is more advantageous for preventing enamel demineralization.
Nevertheless, recent studies employing bracket materials and multispecies biofilms have aimed to determine which brackets are more susceptible to enamel demineralization.19,46 These limited studies have indicated that bracket materials with rougher surfaces and higher surface-free energy tend to increase biofilm formation and the proportion of MS in the biofilm (Figure 4D–4F).19,46 This is attributed to rougher surfaces providing an increased area for bacterial adhesion and protection of biofilms from shear forces, while higher surface-free energy thermodynamically facilitates bacterial adhesion and biofilm formation.11,29,47 Additionally, the composition of cariogenic bacteria in biofilms varies based on caries activity, with a higher proportion of S. mutans found in biofilms from individuals with high caries activity.46 Considering that differences in bracket surface roughness and surface-free energy depend on surface treatment, precise surface processing is essential to create smoother surfaces with lower surface-free energy to prevent biofilm formation and reduce the proportion of MS in biofilms around orthodontic brackets.
However, the shape and size of brackets, rather than the material itself, can influence bacterial adhesion and biofilm formation.15,16 Therefore, opting for smaller-sized brackets with simpler designs is recommended to minimize bacterial adhesion and biofilm formation. Self-ligating brackets, characterized by their reduced size and complexity through the elimination of stainless steel or elastomeric ligatures, may offer potential advantages for oral hygiene by lowering bacterial adhesion and biofilm formation. However, there remains a need for further research to evaluate the effects of self-ligating brackets on biofilm formation and periodontal status.48 Future investigations on self-ligating brackets should address these aspects in relation to biofilm formation and enamel demineralization.
Enamel demineralization is most commonly observed at the interface between enamel and orthodontic adhesives.49,50 This specific area is susceptible to rapid growth and colonization by oral bacteria due to surface roughening caused by toothbrushing and mechanical erosion, which can induce rapid growth and colonization of oral bacteria. In addition, orthodontic adhesives release leachable monomers that promote bacterial adhesion and biofilm formation.39,40 Moreover, adhesives tend to exhibit rougher surfaces and higher surface-free energy compared to bracket materials (Figure 4). These factors create a more favorable environment for bacterial adhesion and biofilm formation on adhesive surfaces than on bracket surfaces.10,19 As such, orthodontic adhesives may play a more significant role in enamel demineralization than orthodontic brackets.
Various methods have been explored to prevent biofilm formation and enamel demineralization around orthodontic adhesives. Among these, incorporating fluoride into orthodontic adhesives has garnered attention due to fluoride's well-known anti-cariogenic properties. Fluoride can enhance remineralization, inhibit biofilm formation, and hinder microbial growth and metabolism.51-54 Incorporating fluoride into orthodontic adhesives is appealing because it can directly deliver fluoride ions to the marginal interface between enamel and orthodontic appliances, reducing the reliance on patient compliance. Resin-modified glass ionomer (RMGI), compomer, and fluoride-releasing composite materials have been developed for orthodontic adhesives with varying fluoride-releasing capabilities.
Resin-modified glass ionomer has shown the highest fluoride-releasing capacity, followed by compomers and fluoride-releasing composites.55 However, most fluoride-releasing adhesives experience their highest fluoride ion release within the first 24 hours, followed by a low level of long-term fluoride ion release (Figure 6).55,56 This limitation stems from the fact that the low fluoride ion levels may not reach the threshold required for therapeutic effects; therefore, it may be considered a limitation of fluoride-release adhesives in terms of long-term enamel demineralization around orthodontic adhesives.
However, fluoride-releasing materials can absorb fluoride from the surrounding environment, replacing the released fluoride.55,56 Fluoride uptake and re-release properties may help to maintain fluoride levels around adhesives for a long time if fluoride is regularly delivered. Studies using orthodontic adhesives have reported that RMGI’s fluoride-recharging capacity is higher than that of a compomer; however, fluoride-releasing composites have little ability to uptake fluoride (Figure 7).55,56 This is because the site occupied by fluoride is limited in the materials, and the fluoride-recharging capacity is related to the amount of fluoride ions released. Fluoride-recharging capacity is independent of the aging of the materials and is more effective in an environment with high fluoride concentration, frequent fluoride application, and a low pH that reproduces high caries activity.55,56
To maintain fluoride levels around orthodontic adhesives, regular fluoride recharging is recommended, ideally using acid phosphate fluoride gel or a high-concentration fluoride solution of 900 ppm or higher (Figure 7).55,56 However, the strong acidity of acid phosphate fluoride gel can degrade the substrate of the orthodontic adhesives;57 therefore, the recommended way to maintain fluoride levels around orthodontic adhesives may be the combination of RMGI with a high-concentration fluoride solution of 900 ppm or higher. Since fluoride re-release occurs over approximately 3 days following topical fluoride exposure,55 regular biweekly fluoride recharging is advised.
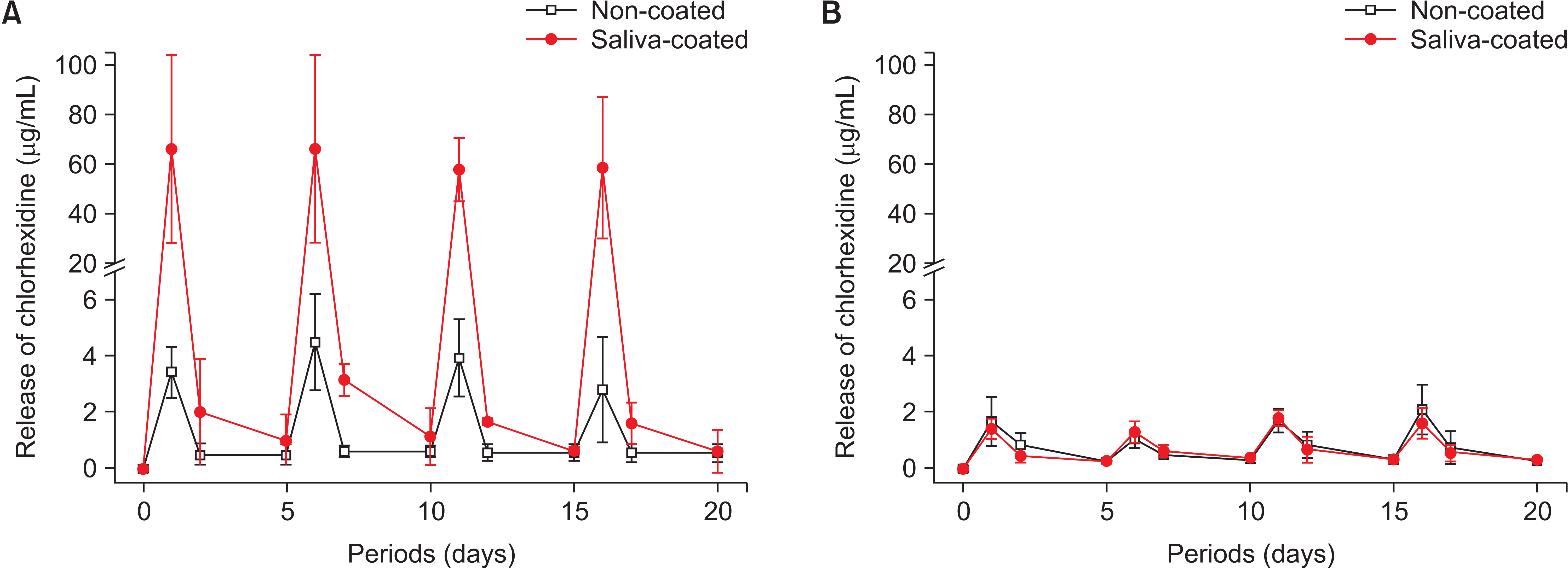
Incorporating CHX into orthodontic adhesives has been explored to develop antimicrobial resin-based materials. However, the incorporation of a high concentration of CHX, required for sufficient antimicrobial activity, can negatively impact the physical and mechanical properties of orthodontic adhesives, resulting in a higher bond failure rate.58,59 Additionally, the released amounts of CHX are not sustained over an extended period.59 Alternatively, CHX’s unique adsorption property onto intraoral surfaces in the oral cavity could be employed to prevent enamel demineralization around orthodontic adhesives.60,61 In particular, composite adhesives can uptake and release a clinically significant amount of CHX for more than 2 days upon exposure to CHX solution (Figure 8).60 Because composite adhesives have not demonstrated the capability to uptake and release a clinically significant amount of fluoride,55,56 regular CHX applications to composite adhesives may effectively prevent enamel demineralization during orthodontic treatment, especially in patients with high caries activity and/or poor oral hygiene.
In addition to fluoride and CHX, various antimicrobial agents, including bioglass, nanoparticles, and quaternary ammonium compounds, have been incorporated into orthodontic adhesives.62-64 However, these new adhesive formulations have some drawbacks, such as reduced mechanical properties and diminished color stability. While in vitro evaluations have shown promising antimicrobial and mechanical properties, most studies have only employed one or two bacterial species for antimicrobial testing without assessing cytotoxicity. Furthermore, their long-term clinical performance has yet to be established. Comprehensive, well-controlled clinical trials with long-term follow-up are essential for validating the clinical utility of orthodontic adhesives containing antimicrobial agents.
Another factor to consider in the relationship between orthodontic adhesives and enamel demineralization is biofilm formation around these adhesives. Several studies have highlighted significant differences in MS adhesion and the composition of MS in biofilms among different adhesive types. These findings indicate that MS adheres more readily to RMGI than to composites.8,12 Additionally, the proportion of MS in biofilms formed on RMGI is higher than on compomers and composites.17 This may be because, despite its fluoride-releasing characteristics, RMGI (Figure 4I) has a rougher surface and higher surface-free energy than composites (Figure 4G) and compomers (Figure 4H), which may have a significant effect on the initial colonization and biofilm formation of MS.12,17 Therefore, caution is advised when using RMGI as a bonding agent during long-term orthodontic treatment, despite its fluoride-releasing and fluoride-recharging capabilities.
Enamel demineralization, often resulting in white spot formation, remains a significant challenge in orthodontic practice. Recent advancements in orthodontic microbial research have shed light on how orthodontic appliances and procedures can promote biofilm formation and elevate the proportion of cariogenic MS in these biofilms by disrupting biofilm balance. However, our understanding of the intricate interactions between cariogenic bacteria and orthodontic appliances, leading to enamel demineralization, remains limited. Given that controlling the virulent activities of MS within biofilms is a promising strategy for preventing enamel demineralization, it is imperative to conduct further research aimed at curbing the persistence of cariogenic MS in biofilms. This research should explore the modulation of biocompatibilities and surface characteristics of orthodontic materials, providing the scientific foundation needed for effective enamel demineralization prevention.
Furthermore, the introduction of next-generation sequencing (NGS) into microbiology has made it possible to obtain the complete bacterial genome in a single sequence run. This breakthrough enables the comprehensive examination of ecological and populational shifts in the entire oral microbiome after the placement of fixed orthodontic appliances. Future studies leveraging NGS will offer valuable insights into the changes occurring in the oral ecosystem associated with enamel demineralization, extending beyond microbial alterations within biofilms.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: SJA. Data curation: JSA, BSL. Formal analysis: JSA, SJA. Investigation: JSA, BSL, SJA. Methodology: JSA, BSL, SJA. Project administration: JSA, BSL, SJA. Supervision: SJA. Validation: BSL, SJA. Visualization: JSA, BSL, SJA. Writing–original draft: SJA. Writing–review & editing: JSA, BSL, SJA.
REFERENCES
1. Mizrahi E. 1982; Enamel demineralization following orthodontic treatment. Am J Orthod. 82:62–7. https://doi.org/10.1016/0002-9416(82)90548-6. DOI: 10.1016/0002-9416(82)90548-6. PMID: 6984291.

2. Lucchese A, Gherlone E. 2013; Prevalence of white-spot lesions before and during orthodontic treatment with fixed appliances. Eur J Orthod. 35:664–8. https://doi.org/10.1093/ejo/cjs070. DOI: 10.1093/ejo/cjs070. PMID: 23045306.

3. Sharab L, Loss C, Jensen D, Kluemper GT, Alotaibi M, Nagaoka H. 2023; Prevalence of white spot lesions and gingival index during orthodontic treatment in an academic setting. Am J Orthod Dentofacial Orthop. 163:835–42. https://doi.org/10.1016/j.ajodo.2022.08.023. DOI: 10.1016/j.ajodo.2022.08.023. PMID: 36720655.

4. Sonesson M, Bergstrand F, Gizani S, Twetman S. 2017; Management of post-orthodontic white spot lesions: an updated systematic review. Eur J Orthod. 39:116–21. https://doi.org/10.1093/ejo/cjw023. DOI: 10.1093/ejo/cjw023. PMID: 27030284.

5. Ulukapi H, Koray F, Efes B. 1997; Monitoring the caries risk of orthodontic patients. Quintessence Int. 28:27–9. https://pubmed.ncbi.nlm.nih.gov/10332351/. PMID: 10332351.
6. Babaahmady KG, Challacombe SJ, Marsh PD, Newman HN. 1998; Ecological study of Streptococcus mutans, Streptococcus sobrinus and Lactobacillus spp. at sub-sites from approximal dental plaque from children. Caries Res. 32:51–8. https://doi.org/10.1159/000016430. DOI: 10.1159/000016430. PMID: 9438572.
7. Ahn SJ, Lim BS, Yang HC, Chang YI. 2005; Quantitative analysis of the adhesion of cariogenic streptococci to orthodontic metal brackets. Angle Orthod. 75:666–71. https://pubmed.ncbi.nlm.nih.gov/16097239/. DOI: 10.1043/0003-3219(2005)75[666:QAOTAO]2.0.CO;2. PMID: 16097239.
8. Ahn SJ, Lim BS, Lee YK, Nahm DS. 2006; Quantitative determination of adhesion patterns of cariogenic streptococci to various orthodontic adhesives. Angle Orthod. 76:869–75. https://pubmed.ncbi.nlm.nih.gov/17029524/. DOI: 10.1043/0003-3219(2006)076[0869:QDOAPO]2.0.CO;2. PMID: 17029524.
9. Ahn SJ, Lee SJ, Lim BS, Nahm DS. 2007; Quantitative determination of adhesion patterns of cariogenic streptococci to various orthodontic brackets. Am J Orthod Dentofacial Orthop. 132:815–21. https://doi.org/10.1016/j.ajodo.2005.09.034. DOI: 10.1016/j.ajodo.2005.09.034. PMID: 18068602.

10. Lim BS, Lee SJ, Lee JW, Ahn SJ. 2008; Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. Am J Orthod Dentofacial Orthop. 133:882–8. https://doi.org/10.1016/j.ajodo.2006.07.027. DOI: 10.1016/j.ajodo.2006.07.027. PMID: 18538253.

11. Lee SP, Lee SJ, Lim BS, Ahn SJ. 2009; Surface characteristics of orthodontic materials and their effects on adhesion of mutans streptococci. Angle Orthod. 79:353–60. https://doi.org/10.2319/021308-88.1. DOI: 10.2319/021308-88.1. PMID: 19216592.

12. Ahn SJ, Lim BS, Lee SJ. 2010; Surface characteristics of orthodontic adhesives and effects on streptococcal adhesion. Am J Orthod Dentofacial Orthop. 137:489–95. discussion 13Ahttps://doi.org/10.1016/j.ajodo.2008.05.015. DOI: 10.1016/j.ajodo.2008.05.015. PMID: 20362908.

13. Ahn SJ, Cho EJ, Oh SS, Lim BS. 2012; The effects of orthodontic bonding steps on biofilm formation of Streptococcus mutans in the presence of saliva. Acta Odontol Scand. 70:504–10. https://doi.org/10.3109/00016357.2011.640277. DOI: 10.3109/00016357.2011.640277. PMID: 22181697.

14. Park JW, Song CW, Jung JH, Ahn SJ, Ferracane JL. 2012; The effects of surface roughness of composite resin on biofilm formation of Streptococcus mutans in the presence of saliva. Oper Dent. 37:532–9. https://doi.org/10.2341/11-371-L. DOI: 10.2341/11-371-L. PMID: 22339385.

15. Jung WS, Kim H, Park SY, Cho EJ, Ahn SJ. 2014; Quantitative analysis of changes in salivary mutans streptococci after orthodontic treatment. Am J Orthod Dentofacial Orthop. 145:603–9. https://doi.org/10.1016/j.ajodo.2013.12.025. DOI: 10.1016/j.ajodo.2013.12.025. PMID: 24785924.

16. Jung WS, Yang IH, Lim WH, Baek SH, Kim TW, Ahn SJ. 2015; Adhesion of mutans streptococci to self-ligating ceramic brackets: in vivo quantitative analysis with real-time polymerase chain reaction. Eur J Orthod. 37:565–9. https://doi.org/10.1093/ejo/cju090. DOI: 10.1093/ejo/cju090. PMID: 25564502.

17. An JS, Kim K, Cho S, Lim BS, Ahn SJ. 2017; Compositional differences in multi-species biofilms formed on various orthodontic adhesives. Eur J Orthod. 39:528–33. https://doi.org/10.1093/ejo/cjw089. DOI: 10.1093/ejo/cjw089. PMID: 28339597.

18. Jeon DM, An JS, Lim BS, Ahn SJ. 2020; Orthodontic bonding procedures significantly influence biofilm composition. Prog Orthod. 21:14. https://doi.org/10.1186/s40510-020-00314-8. DOI: 10.1186/s40510-020-00314-8. PMID: 32476070. PMCID: PMC7261716. PMID: 8beaedc11b354e41ac8187cdefaefb8f.

19. Park SH, Kim K, Cho S, Chung DH, Ahn SJ. 2022; Variation in adhesion of Streptococcus mutans and Porphyromonas gingivalis in saliva-derived biofilms on raw materials of orthodontic brackets. Korean J Orthod. 52:278–86. https://doi.org/10.4041/kjod21.283. DOI: 10.4041/kjod21.283. PMID: 35678009. PMCID: PMC9314218.

20. Jung WS, Kim K, Cho S, Ahn SJ. 2016; Adhesion of periodontal pathogens to self-ligating orthodontic brackets: an in-vivo prospective study. Am J Orthod Dentofacial Orthop. 150:467–75. https://doi.org/10.1016/j.ajodo.2016.02.023. DOI: 10.1016/j.ajodo.2016.02.023. PMID: 27585775.

21. Kim K, Jung WS, Cho S, Ahn SJ. 2016; Changes in salivary periodontal pathogens after orthodontic treatment: an in vivo prospective study. Angle Orthod. 86:998–1003. https://doi.org/10.2319/070615-450.1. DOI: 10.2319/070615-450.1. PMCID: PMC8597347.

22. Ahn SJ, Kho HS, Kim KK, Nahm DS. 2003; Adhesion of oral streptococci to experimental bracket pellicles from glandular saliva. Am J Orthod Dentofacial Orthop. 124:198–205. https://doi.org/10.1016/s0889-5406(03)00346-9. DOI: 10.1016/S0889-5406(03)00346-9. PMID: 12923517.

23. Lee SJ, Kho HS, Lee SW, Yang WS. 2001; Experimental salivary pellicles on the surface of orthodontic materials. Am J Orthod Dentofacial Orthop. 119:59–66. https://doi.org/10.1067/mod.2001.110583. DOI: 10.1067/mod.2001.110583. PMID: 11174541.

24. Ahn SJ, Kho HS, Lee SW, Nahm DS. 2002; Roles of salivary proteins in the adherence of oral streptococci to various orthodontic brackets. J Dent Res. 81:411–5. https://doi.org/10.1177/154405910208100611. DOI: 10.1177/154405910208100611. PMID: 12097434.

25. Ahn SJ, Ahn SJ, Wen ZT, Brady LJ, Burne RA. 2008; Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva. Infect Immun. 76:4259–68. https://doi.org/10.1128/IAI.00422-08. DOI: 10.1128/IAI.00422-08. PMID: 18625741. PMCID: PMC2519434.

26. Burne RA, Quivey RG Jr, Marquis RE. 1999; Physiologic homeostasis and stress responses in oral biofilms. Methods Enzymol. 310:441–60. https://doi.org/10.1016/s0076-6879(99)10035-1. DOI: 10.1016/S0076-6879(99)10035-1. PMID: 10547811.

27. ten Cate JM. 2006; Biofilms, a new approach to the microbiology of dental plaque. Odontology. 94:1–9. https://doi.org/10.1007/s10266-006-0063-3. DOI: 10.1007/s10266-006-0063-3. PMID: 16998612.

28. Burne RA. 1998; Oral streptococci... products of their environment. J Dent Res. 77:445–52. https://doi.org/10.1177/00220345980770030301. DOI: 10.1177/00220345980770030301. PMID: 9496917.

29. Park JW, An JS, Lim WH, Lim BS, Ahn SJ. 2019; Microbial changes in biofilms on composite resins with different surface roughness: an in vitro study with a multispecies biofilm model. J Prosthet Dent. 122:493.e1–493.e8. https://doi.org/10.1016/j.prosdent.2019.08.009. DOI: 10.1016/j.prosdent.2019.08.009. PMID: 31648793.

30. Bowen WH, Burne RA, Wu H, Koo H. 2018; Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26:229–42. https://doi.org/10.1016/j.tim.2017.09.008. DOI: 10.1016/j.tim.2017.09.008. PMID: 29097091. PMCID: PMC5834367.

31. Jakubovics NS, Goodman SD, Mashburn-Warren L, Stafford GP, Cieplik F. 2021; The dental plaque biofilm matrix. Periodontol 2000. 86:32–56. https://doi.org/10.1111/prd.12361. DOI: 10.1111/prd.12361. PMID: 33690911. PMCID: PMC9413593.

32. Ahn SJ, Ahn SJ, Browngardt CM, Burne RA. 2009; Changes in biochemical and phenotypic properties of Streptococcus mutans during growth with aeration. Appl Environ Microbiol. 75:2517–27. https://doi.org/10.1128/AEM.02367-08. DOI: 10.1128/AEM.02367-08. PMID: 19251884. PMCID: PMC2675223.

33. Yang IH, Lim BS, Park JR, Hyun JY, Ahn SJ. 2011; Effect of orthodontic bonding steps on the initial adhesion of mutans streptococci in the presence of saliva. Angle Orthod. 81:326–33. https://pubmed.ncbi.nlm.nih.gov/21208087/. DOI: 10.2319/062210-343.1. PMID: 21208087. PMCID: PMC8925257.

34. O'Reilly MM, Featherstone JD. 1987; Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 92:33–40. https://doi.org/10.1016/0889-5406(87)90293-9. DOI: 10.1016/0889-5406(87)90293-9. PMID: 3300270.
35. Collys K, Cleymaet R, Coomans D, Slop D. 1991; Acid-etched enamel surfaces after 24 h exposure to calcifying media in vitro and in vivo. J Dent. 19:230–5. https://doi.org/10.1016/0300-5712(91)90124-h. DOI: 10.1016/0300-5712(91)90124-H. PMID: 1787212.

36. Sideridou I, Tserki V, Papanastasiou G. 2002; Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 23:1819–29. https://doi.org/10.1016/s0142-9612(01)00308-8. DOI: 10.1016/S0142-9612(01)00308-8. PMID: 11950052.

37. Polydorou O, König A, Hellwig E, Kümmerer K. 2009; Long-term release of monomers from modern dental-composite materials. Eur J Oral Sci. 117:68–75. https://doi.org/10.1111/j.1600-0722.2008.00594.x. DOI: 10.1111/j.1600-0722.2008.00594.x. PMID: 19196321.

38. Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. 2010; Biofilm formation on dental restorative and implant materials. J Dent Res. 89:657–65. https://doi.org/10.1177/0022034510368644. DOI: 10.1177/0022034510368644. PMID: 20448246.

39. Kim K, An JS, Lim BS, Ahn SJ. 2019; Effect of bisphenol A glycol methacrylate on virulent properties of Streptococcus mutans UA159. Caries Res. 53:84–95. https://doi.org/10.1159/000490197. DOI: 10.1159/000490197. PMID: 29961075.

40. Kim K, Kim JN, Lim BS, Ahn SJ. 2021; Urethane dimethacrylate influences the cariogenic properties of Streptococcus mutans. Materials (Basel). 14:1015. https://doi.org/10.3390/ma14041015. DOI: 10.3390/ma14041015. PMID: 33669956. PMCID: PMC7924865. PMID: 7aa8acbeae094385802eca3411e5a4ac.

41. Chung SH, Cho S, Kim K, Lim BS, Ahn SJ. 2017; Antimicrobial and physical characteristics of orthodontic primers containing antimicrobial agents. Angle Orthod. 87:307–12. https://doi.org/10.2319/052516-416.1. DOI: 10.2319/052516-416.1. PMID: 27598781. PMCID: PMC8384363.

42. Özel MB, Tüzüner T, Güplü ZA, Coleman NJ, Hurt AP, Buruk CK. 2017; The antibacterial activity and release of quaternary ammonium compounds in an orthodontic primer. Acta Odontol Latinoam. 30:141–8. https://pubmed.ncbi.nlm.nih.gov/29750238/.
43. Oz AZ, Oz AA, Yazicioglu S, Sancaktar O. 2019; Effectiveness of an antibacterial primer used with adhesive-coated brackets on enamel demineralization around brackets: an in vivo study. Prog Orthod. 20:15. https://doi.org/10.1186/s40510-019-0271-3. DOI: 10.1186/s40510-019-0271-3. PMID: 30982931. PMCID: PMC6462439. PMID: 2c219361e6f640369ff0d2e6bf1c159e.

44. Faltermeier A, Bürgers R, Rosentritt M. 2008; Bacterial adhesion of Streptococcus mutans to esthetic bracket materials. Am J Orthod Dentofacial Orthop. 133(4 Suppl):S99–103. https://doi.org/10.1016/j.ajodo.2007.03.024. DOI: 10.1016/j.ajodo.2007.03.024. PMID: 18407028.

45. Papaioannou W, Gizani S, Nassika M, Kontou E, Nakou M. 2007; Adhesion of Streptococcus mutans to different types of brackets. Angle Orthod. 77:1090–5. https://doi.org/10.2319/091706-375.1. DOI: 10.2319/091706-375.1. PMID: 18004916.

46. Lim BS, Kim BH, Shon WJ, Ahn SJ. 2020; Effects of caries activity on compositions of mutans streptococci in saliva-induced biofilm formed on bracket materials. Materials (Basel). 13:4764. https://doi.org/10.3390/ma13214764. DOI: 10.3390/ma13214764. PMID: 33114489. PMCID: PMC7663262.

47. Ionescu A, Wutscher E, Brambilla E, Schneider-Feyrer S, Giessibl FJ, Hahnel S. 2012; Influence of surface properties of resin-based composites on in vitro Streptococcus mutans biofilm development. Eur J Oral Sci. 120:458–65. https://doi.org/10.1111/j.1600-0722.2012.00983.x. DOI: 10.1111/j.1600-0722.2012.00983.x. PMID: 22985005.

48. Skilbeck MG, Mei L, Mohammed H, Cannon RD, Farella M. 2022; The effect of ligation methods on biofilm formation in patients undergoing multi-bracketed fixed orthodontic therapy - a systematic review. Orthod Craniofac Res. 25:14–30. https://doi.org/10.1111/ocr.12503. DOI: 10.1111/ocr.12503. PMID: 34042260.

49. Gwinnett AJ, Ceen RF. 1979; Plaque distribution on bonded brackets: a scanning microscope study. Am J Orthod. 75:667–77. https://doi.org/10.1016/0002-9416(79)90098-8. DOI: 10.1016/0002-9416(79)90098-8. PMID: 377979.

50. Sukontapatipark W, el-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. 2001; Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod. 23:475–84. https://doi.org/10.1093/ejo/23.5.475. DOI: 10.1093/ejo/23.5.475. PMID: 11668867.

51. Carey CM. 2014; Focus on fluorides: update on the use of fluoride for the prevention of dental caries. J Evid Based Dent Pract. 14 Suppl:95–102. https://doi.org/10.1016/j.jebdp.2014.02.004. DOI: 10.1016/j.jebdp.2014.02.004. PMID: 24929594. PMCID: PMC4058575.

52. Griffin SO, Regnier E, Griffin PM, Huntley V. 2007; Effectiveness of fluoride in preventing caries in adults. J Dent Res. 86:410–5. https://doi.org/10.1177/154405910708600504. DOI: 10.1177/154405910708600504. PMID: 17452559.

53. O'Mullane DM, Baez RJ, Jones S, Lennon MA, Petersen PE, Rugg-Gunn AJ, et al. 2016; Fluoride and Oral Health. Community Dent Health. 33:69–99. https://doi.org/10.1922/CDH_3707O'Mullane31. PMID: 27352462.
54. Ogaard B. 1990; Effects of fluoride on caries development and progression in vivo. J Dent Res. 69 Spec No:813–9. discussion 820–3. https://doi.org/10.1177/00220345900690S155. DOI: 10.1177/00220345900690S155. PMID: 2179345.

55. Ahn SJ, Lee SJ, Lee DY, Lim BS. 2011; Effects of different fluoride recharging protocols on fluoride ion release from various orthodontic adhesives. J Dent. 39:196–201. https://doi.org/10.1016/j.jdent.2010.12.003. DOI: 10.1016/j.jdent.2010.12.003. PMID: 21147194.

56. Lim BS, Lee SJ, Lim YJ, Ahn SJ. 2011; Effects of periodic fluoride treatment on fluoride ion release from fresh orthodontic adhesives. J Dent. 39:788–94. https://doi.org/10.1016/j.jdent.2011.08.011. DOI: 10.1016/j.jdent.2011.08.011. PMID: 21896303.

57. Fukazawa M, Matsuya S, Yamane M. 1990; The mechanism for erosion of glass-ionomer cements in organic-acid buffer solutions. J Dent Res. 69:1175–9. https://doi.org/10.1177/00220345900690051001. DOI: 10.1177/00220345900690051001. PMID: 2335651.

58. Dionysopoulos D. 2016; Effect of digluconate chlorhexidine on bond strength between dental adhesive systems and dentin: a systematic review. J Conserv Dent. 19:11–6. https://doi.org/10.4103/0972-0707.173185. DOI: 10.4103/0972-0707.173185. PMID: 26957786. PMCID: PMC4760005.

59. Chen L, Suh BI, Yang J. 2018; Antibacterial dental restorative materials: a review. Am J Dent. 31(Sp Is B):6B–12B. https://pubmed.ncbi.nlm.nih.gov/31099206/. PMID: 31099206.
60. Lim BS, Cheng Y, Lee SP, Ahn SJ. 2013; Chlorhexidine release from orthodontic adhesives after topical chlorhexidine treatment. Eur J Oral Sci. 121(3 Pt 1):211–7. https://doi.org/10.1111/eos.12033. DOI: 10.1111/eos.12033. PMID: 23659245.

61. Ryu HS, Kim YI, Lim BS, Lim YJ, Ahn SJ. 2015; Chlorhexidine uptake and release from modified titanium surfaces and its antimicrobial activity. J Periodontol. 86:1268–75. https://doi.org/10.1902/jop.2015.150075. DOI: 10.1902/jop.2015.150075. PMID: 26156675.

62. Ahn SJ, Lee SJ, Kook JK, Lim BS. 2009; Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 25:206–13. https://doi.org/10.1016/j.dental.2008.06.002. DOI: 10.1016/j.dental.2008.06.002. PMID: 18632145.

63. Liu Y, Zhang L, Niu LN, Yu T, Xu HHK, Weir MD, et al. 2018; Antibacterial and remineralizing orthodontic adhesive containing quaternary ammonium resin monomer and amorphous calcium phosphate nanoparticles. J Dent. 72:53–63. https://doi.org/10.1016/j.jdent.2018.03.004. DOI: 10.1016/j.jdent.2018.03.004. PMID: 29534887.

64. Degrazia FW, Altmann ASP, Ferreira CJ, Arthur RA, Leitune VCB, Samuel SMW, et al. 2019; Evaluation of an antibacterial orthodontic adhesive incorporated with niobium-based bioglass: an in situ study. Braz Oral Res. 33:e010. https://doi.org/10.1590/1807-3107bor-2019.vol33.0010. DOI: 10.1590/1807-3107bor-2019.vol33.0010. PMID: 30892409. PMID: f7f278c492e14af38aed3a5d7e673d94.

Figure 1
Biofilm formation around orthodontic appliances. After the formation of salivary pellicles, the initial colonizers are attached, and then step-by-step biofilm maturation is achieved by the interaction between various microorganisms.
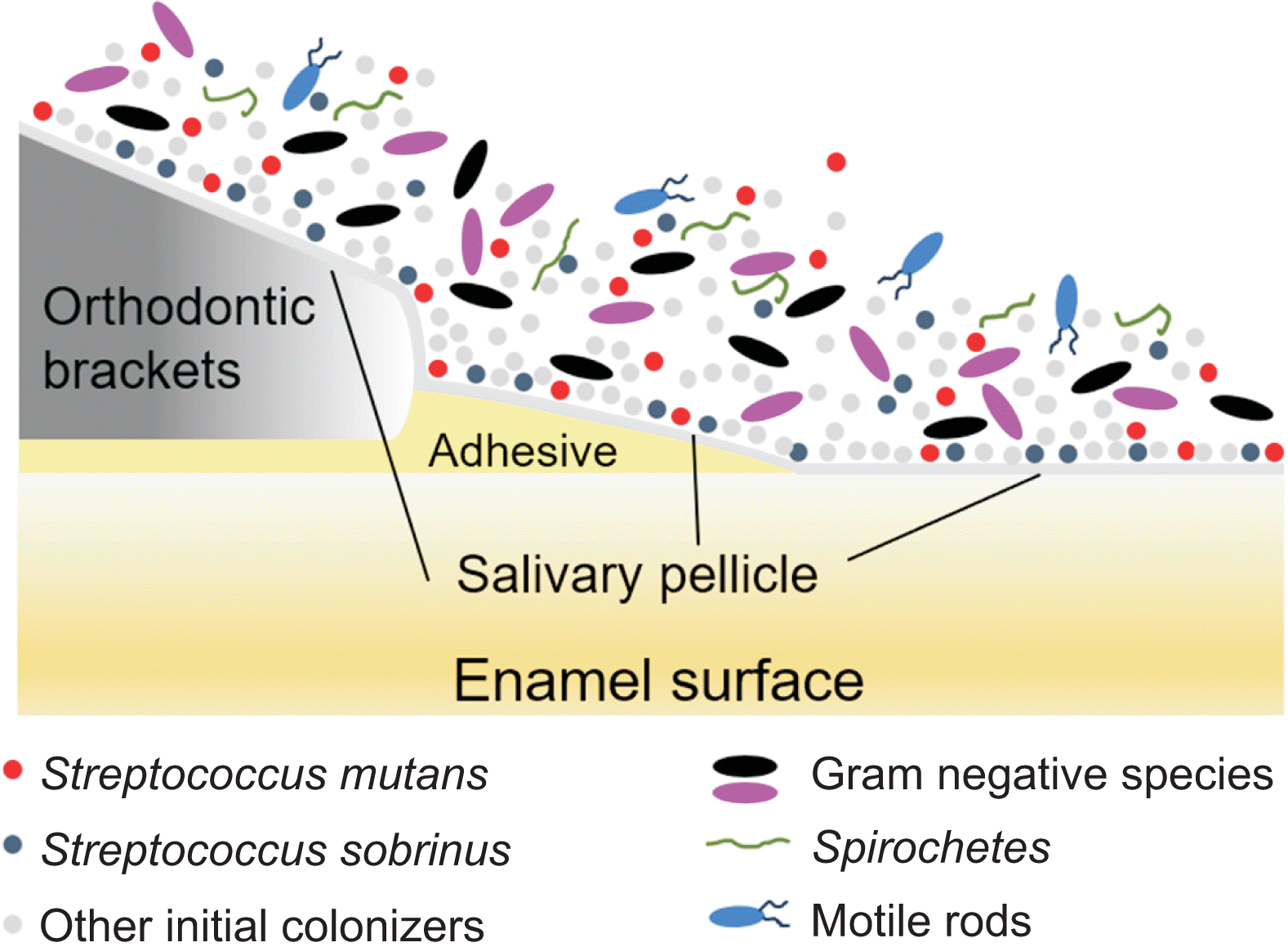
Figure 2
Biofilm formation of Streptococcus mutans (S. mutans) in the absence (A, B) and presence of sucrose (C, D). For biofilm formation, S. mutans UA159 was grown in a semi-defined biofilm medium with 20 mM glucose (A, B) or sucrose (C, D) for 24 hours after saliva-coating. The images were taken using a confocal laser scanning microscope (CLSM) at 200× magnification after biofilms were generated on eight-well Lab-Tek Chamber Permanox slides (Nagle Nunc International, Rochester, NY, USA). The bacterial cells (green) were stained with SYTO 13 fluorescent nucleic acid stain (Thermo Fisher Scientific, Madison, WI, USA) for 20 minutes before CLSM analyses. Biofilm developed by S. mutans was denser and deeper in the presence of sucrose (C, D) than in the absence of sucrose (A, B) due to the extracellular polysaccharide matrices.
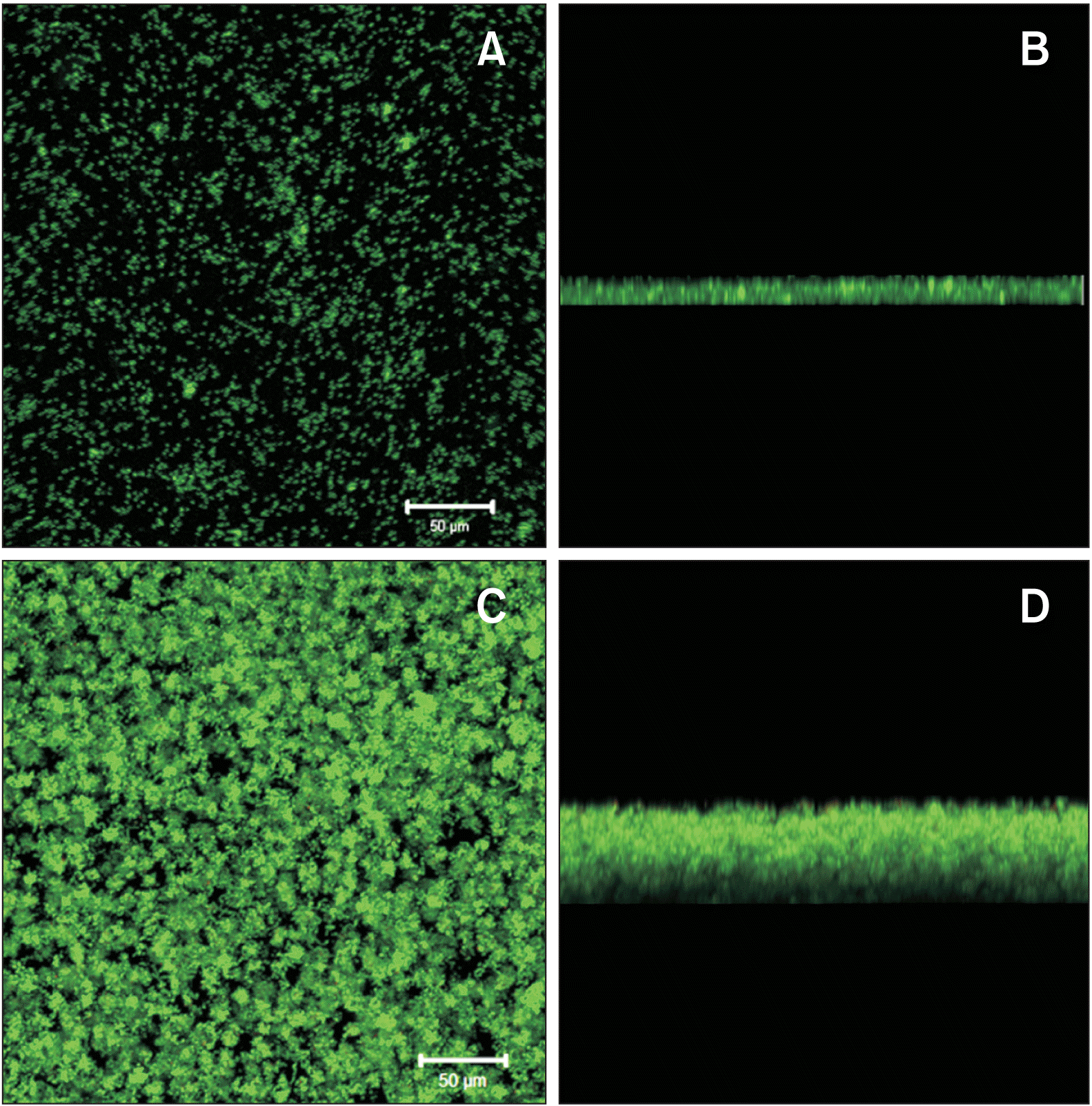
Figure 3
Effects of orthodontic bonding procedures on biofilm formation Streptococcus mutans (S. mutans): A, E, untreated hydroxyapatite surface; B, F, etched hydroxyapatite surface; C, G, primed surface; D, H, adhesive surface. For biofilm formation, S. mutans UA159 was grown in a semi-defined biofilm medium with 20 mM glucose A–D, or sucrose E–H, for 24 hours after saliva-coating. The biofilm images were taken using a scanning electron microscope at 3,000× magnification. The presence of sucrose significantly promoted the biofilm formation of S. mutans, regardless of the orthodontic bonding procedures.
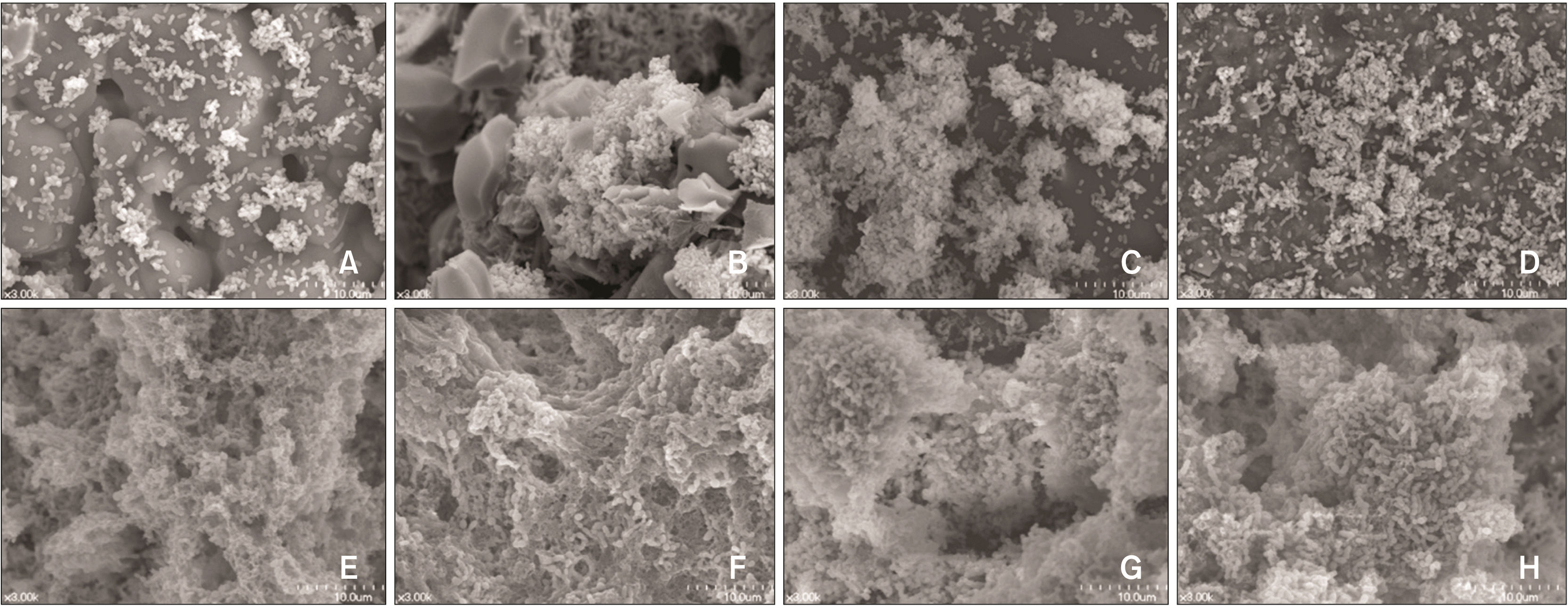
Figure 4
Scanning electron microscope images of the bovine enamel A–C, bracket materials D–F, and orthodontic adhesives G–I, at ×3,000 magnification: A, untreated surface; B, etched surface; C, primed surface; D, monocrystalline alumina (Hubit Co., Seoul, Korea); E, stainless steel (Hubit); F, polycarbonate (Hubit); G, composite adhesive (Tansbond XT, 3M, Monrovia, CA, USA); H, compomer (Tansbond Plus, 3M); I, resin-modified glass ionomer (Fuji Otho LC, GC, Tokyo, Japan). The adhesive surfaces G-I, are rougher than the bracket surfaces D-F, due to the filler and/or glass particles contained in the adhesives. In particular, the resin-modified glass ionomer has the roughest surface due to its larger glass particles I.
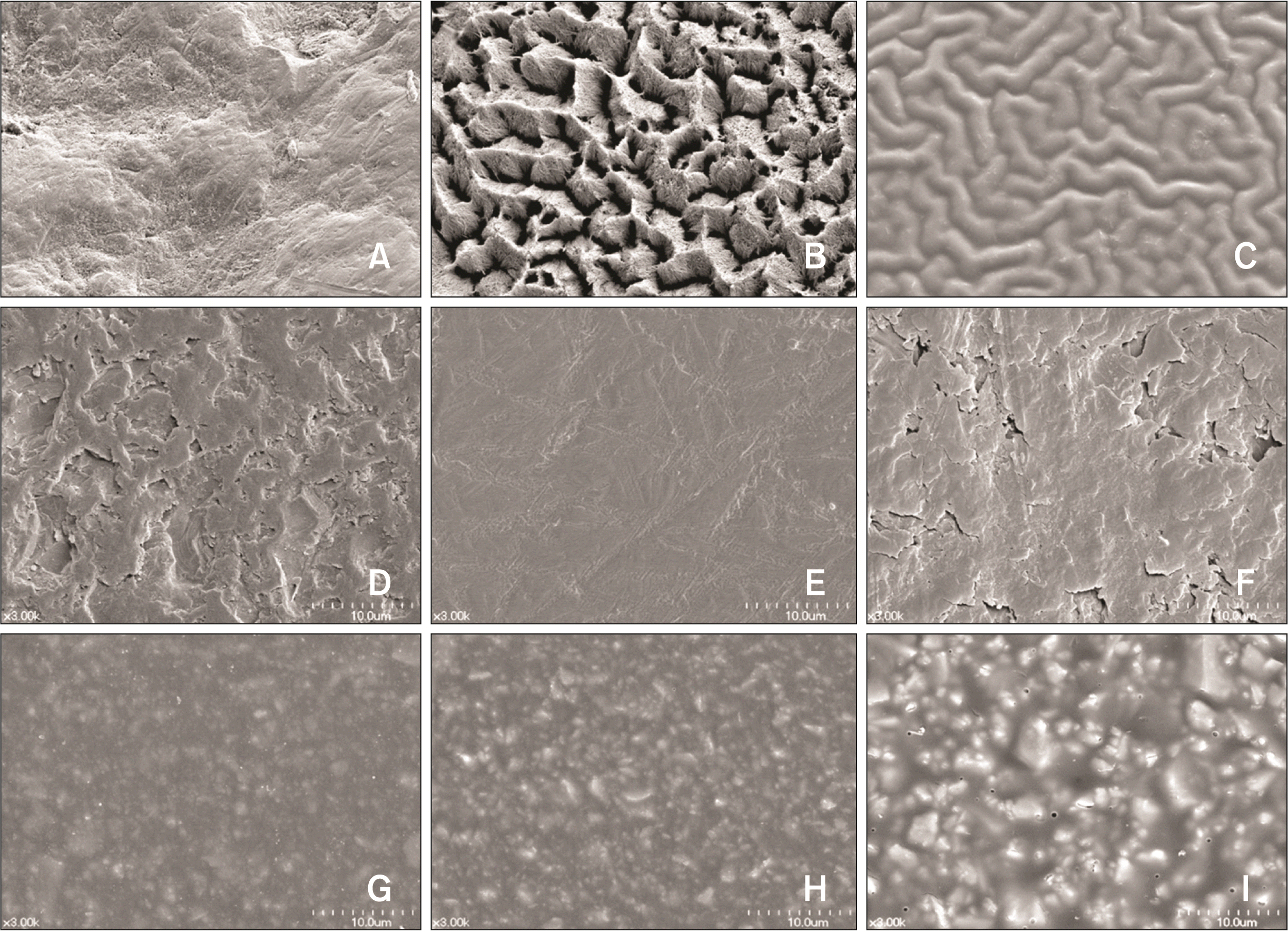
Figure 5
Effects of bisphenol A glycidyl methacrylate (bis-GMA) (B, E) and urethane dimethacrylate (UDMA) (C, F) on biofilm formation of Streptococcus mutans (S. mutans) in the absence (A–C) and presence of sucrose (D–F). For biofilm formation, S. mutans UA159 was grown in a semi-defined biofilm medium with 20 mM glucose or sucrose for 24 hours after saliva-coating. The images were taken using a confocal laser scanning microscope at ×1,000 magnification after biofilms were generated on 8-well Lab-Tek Chamber Permanox slides (Nagle Nunc International, Rochester, NY, USA). The bacterial cells (green) and polysaccharide biofilm matrices (red) were stained with SYTO 13 fluorescent nucleic acid stain (Thermo Fisher Scientific, Madison, WI, USA) and Alexa Flour 647-labeled dextran conjugate (Thermo Fisher Scientific), respectively. Bis-GMA (B, E) and UDMA (E, F) significantly increased the biofilm formation of S. mutans compared to control (A, D). In particular, both bis-GMA (E) and UDMA (F) significantly enhanced the biofilm formation of S. mutans in the presence of sucrose by facilitating the production of extracellular polysaccharide matrices.
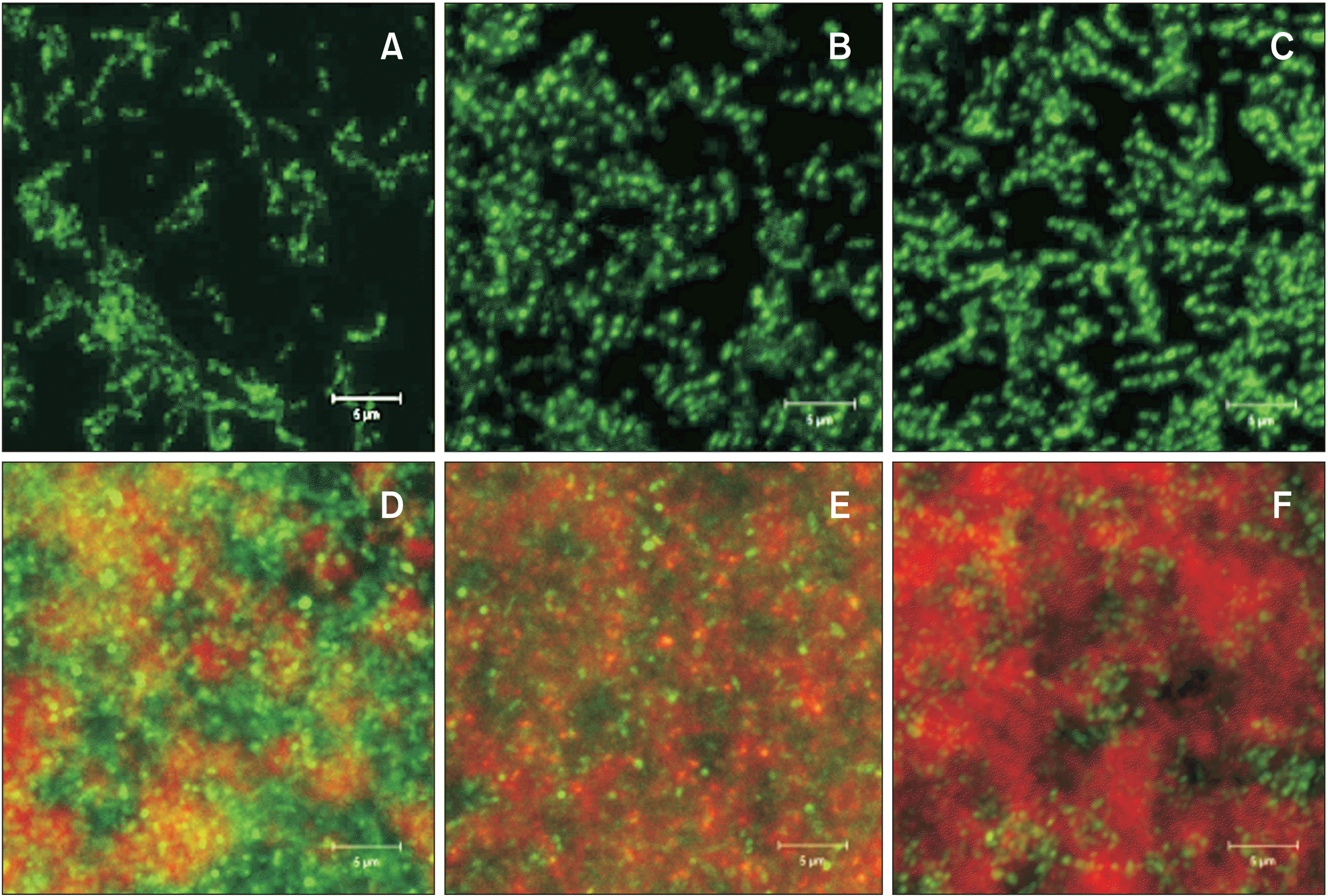
Figure 6
The amount of fluoride ion released from a nonfluoride-releasing composite (Transbond XT, 3M, Monrovia, CA, USA), a fluoride-releasing composite (Light Bond, Reliance Orthodontics, Itasca, IL, USA), a compomer (Transbond Plus, 3M), and 2 resin-modified glass ionomers (Multi-Cure, 3M; and Fuji Ortho LC, GC, Tokyo, Japan). The highest level of fluoride ion release appears during the first 24 hours after exposure, followed by a low level of long-term fluoride ion release.
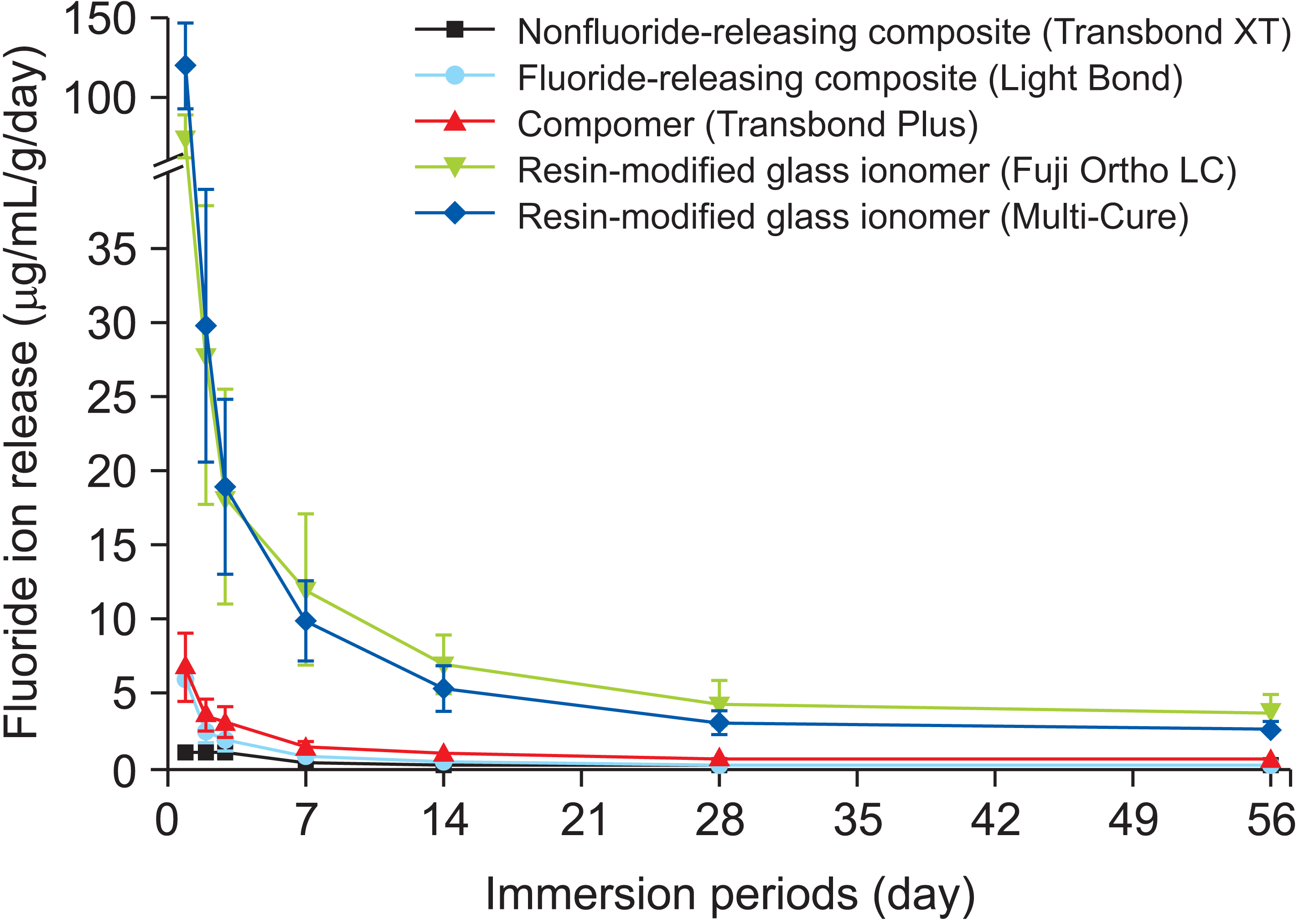
Figure 7
Fluoride uptake and re-release from various orthodontic adhesives after topical fluoride treatment for 1 minute: A, deionized water; B, acidulate phosphate fluoride gel (Oral-B, Belmont, CA, USA); C, a fluoridated dentifrice containing 1,000 ppm sodium fluoride (Regular Care, Crest, Cincinnati, OH, USA); D, sodium fluoride solution containing 900 ppm fluoride ion. Each fluoride treatment is performed in a 5-day cycle.
Figure 8
Chlorhexidine release from orthodontic adhesives in non-coated and saliva-coated groups after periodic exposure to a 1.0% chlorhexidine digluconate solution (Sigma-Aldrich, St. Louis, MO, USA); A, composite adhesive (Transbond XT, 3M, Monrovia, CA, USA); B, resin-modified glass ionomer (Fuji Ortho LC, GC, Tokyo, Japan) Each chlorhexidine treatment is performed in a 5-day cycle. Saliva-coating significantly increased chlorhexidine release from the composite adhesive.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download