Dr. Nanyeong Kim: A 75-year-old man visited the clinic complaining of tongue swelling that persisted over one year. The patient stated that he had experienced an unspecified skin rash and reported that the generalized edema had gradually worsened over the past three months. The patient was taking losartan 50 mg quaque die (qd) for hypertension and naftopidil 50 mg qd for benign prostate hyperplasia. He was a past smoker with a history of 40 pack-years and said he frequently drank one bottle of alcohol two to three times a week.
On examination, prominent macroglossia was observed and generalized edema was predominantly seen in the lower limbs (Fig. 1). Complete blood count showed anemia with hemoglobin level of 13.1 g/dL (reference range 14.0–18.0). Routine chemistry confirmed normal liver and renal function. Electrocardiogram showed non-specific intraventricular conduction delay, and chest X-ray showed cardiomegaly with bilateral pleural effusion (Fig. 2). We also found hypercalcemia (serum calcium = 10.9 mg/dL, reference range 8.1–10.6) and elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) level (14,013 pg/mL, reference range 0–125).
Echocardiography revealed diastolic heart failure with preserved apical systolic function and prominent ventricular wall thickening and restrictive cardiomyopathy (Fig. 3). For hypercalcemia, suppressed phosphorous (serum phosphorous = 2.3 mg/dL, reference range 2.5–4.5) and elevated parathyroid hormone-related protein (serum PTHrP = 9.4 mg/dL, reference range 0.50–2.81) were confirmed, suggesting unknown malignancy (Table 1).
Dr. Jinyoung Kim: For possible causes of cancer-associated hypercalcemia, the following differential diagnosis can be considered.1
Osteolytic lesions caused by malignant bone metastasis may cause hypercalcemia. Patients usually complain of localized pain that can be accompanied by bone metastasis, and an osteolytic lesion can be confirmed on imaging studies. However, bone lesions could not be identified in this patient, so this mechanism of hypercalcemia was excluded.
Parathyroid cancer could lead to hypercalcemia mediated by parathyroid hormone (PTH). However, this was ruled out by low PTH in this patient. Some types of hematologic malignancy may increase the active vitamin D level, but hypercalcemia due to vitamin D is accompanied by hypophosphatemia. In malignant tumors, HHM due to elevated PTHrP accounts for up to 80% of cases in hypercalcemia associated with tumors and is mainly reported in solid cancers.2
Contrast-mediated chest and abdominal computed tomography was performed to examine the possibility of any type of malignancy in this patient, but we could not confirm a solid tumor.
Dr. Tae-Jung Kim: In this case, biopsy was performed on the tongue and endocardium. Green birefringence was confirmed under cross-polarized light in the biopsied tissue subjected to Congo Red staining, and the patient was diagnosed with amyloidosis (Fig. 4).
Dr. Youngwoo Jeon: Serum-free lambda light-chain level was elevated to 726.75 mg/L (reference range, 5.71–26.30), and additional work-up was performed in the hematology department under the diagnosis of amyloidosis. Serum and urine immunofixation electrophoresis suggested plasma cell disorder, which reported monoclonal gammopathy and Bence-Jones proteinuria. Bone marrow biopsy showed plasmacytosis, with a 10% burden of plasma cells (Fig. 5).
Dr. Nanyeong Kim: The patient received systemic chemotherapy (Bortezomib, Melphalan, and Prednisone–VMP–regimen) for multiple myeloma3 and showed very good partial response (defined as a difference in free light chain less than 40 mg/L). For two years after treatment, he has not complained of systemic symptoms and is currently maintaining a stable general condition.
Dr. Hyuk-Sang Kwon: Amyloidosis is a very rare disease. What are the symptoms that could indicate this disease?
Dr. Jinyoung Kim: In amyloid light chain (AL) amyloidosis, macroglossia is a pathognomonic feature, although only one-third of patients showed the finding.4 In addition, protein fibrils are deposited in tissues around the heart, kidneys, liver, gastrointestinal tract, and peripheral nerves and can cause various symptoms. Skin lesions with a reticulated pattern may occur due to perivascular invasion, which is sometimes observed in the form of purpura or ecchymoses.5
Dr. Hyuk-Sang Kwon: Is amyloidosis accompanied by multiple myeloma?
Dr. Tong-Yoon Kim: AL amyloidosis is associated with multiple myeloma in 10–15% of cases and may precede or occur concurrently. AL amyloidosis with multiple myeloma reporting greater than 10% bone marrow plasmacytosis can be considered as a similar entity. For such patients, bone marrow plasmacytosis has a very important role in deciding treatment and predicting prognosis.6
Dr. Hyuk-Sang Kwon: Is heart failure common in patients with amyloidosis?
Dr. Chul Soo Park: Cardiac complication is the leading cause of death in amyloidosis. However, the prevalence of cardiac involvement is reported differently depending on the type of amyloidosis. Cardiac involvement occurs in more than 50% of AL amyloidosis, whereas it is very rare in amyloid A protein amyloidosis but is the predominant feature in amyloid transthyretin amyloidosis.4 In the case of cardiomyopathy reported in amyloidosis, thickening of the ventricle wall is characteristic and appears in the form of concentric thickening rather than focal thickening. Systolic dysfunction is uncommon, but diastolic dysfunction is a frequent manifestation. An apical sparing pattern with preserved apex motility is a typical finding.7
Dr. Hyuk-Sang Kwon: What is the mechanism of hypercalcemia observed in patients?
Dr. Jinyoung Kim: Hypercalcemia in multiple myeloma patients is usually caused by osteolytic lesions, which is one of the typical symptoms of multiple myeloma.8 However, prominent bone involvement was not confirmed in this patient, and hypophosphatemia and PTHrP elevation accompanied by hypercalcemia were confirmed. According to a previous study, increased PTHrP is often reported in multiple myeloma, and it has been hypothesized that it may be associated with bone disease progression.9 In addition, the patient had advanced heart failure at the time of diagnosis, which may be a rare occurrence of cardiac-induced PTHrP elevation and consequent hypercalcemia.10
Dr. Ki-Hyun Baek: In this patient case, the elevation of PTHrP continued despite improvement of myeloma after chemotherapy, and a significant association between PTHrP and NT-proBNP levels was confirmed (Table 2). Discussion of this rare case in which hypercalcemia was thought to be caused by heart failure due to infiltrative disease provided an opportunity to explore the various causes of hypercalcemia.
Dr. Hyuk-Sang Kwon: Amyloidosis is a rare disease for which suspicion can foster early diagnosis and improve prognosis. We attempted to share our experience of a characteristic AL amyloidosis accompanied by multiple myeloma. In addition, this case is a rare condition in which hypercalcemia was caused by elevated PTHrP accompanying multiple myeloma, and its presentation will help to increase the understanding of various pathologic causes of hypercalcemia.
ACKNOWLEDGMENTS
The case conference report was prepared from the case conference of the Division of Endocrinology and Metabolism, Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
References
1. Guise TA, Wysolmerski JJ. Cancer-associated hypercalcemia. N Engl J Med. 2022; 386(15):1443–1451. PMID: 35417639.

2. Jin J, Chung JO, Chung MY, Cho DH, Chung DJ. Clinical characteristics, causes and survival in 115 cancer patients with parathyroid hormone related protein-mediated hypercalcemia. J Bone Metab. 2017; 24(4):249–255. PMID: 29259965.

3. Lee SR, Choi H, Lee BH, Kang KW, Yu ES, Kim DS, et al. Modified dose of melphalan-prednisone in multiple myeloma patients receiving bortezomib plus melphalan-prednisone treatment. Korean J Intern Med. 2019; 34(6):1333–1346. PMID: 30360024.

4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016; 387(10038):2641–2654. PMID: 26719234.

5. Ahmad QM, Sultan SJ, Shah IH, Sameem F. Systemic amyloidosis presenting as mucocutaneous bullous lesions. Hematol Oncol Stem Cell Ther. 2009; 2(3):418–421. PMID: 20139056.

6. Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol. 2013; 31(34):4319–4324. PMID: 24145344.

7. Lee SP, Park JB, Kim HK, Kim YJ, Grogan M, Sohn DW. Contemporary imaging diagnosis of cardiac amyloidosis. J Cardiovasc Imaging. 2019; 27(1):1–10. PMID: 30701710.

8. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78(1):21–33. PMID: 12528874.

9. Cafforio P, Savonarola A, Stucci S, De Matteo M, Tucci M, Brunetti AE, et al. PTHrP produced by myeloma plasma cells regulates their survival and pro-osteoclast activity for bone disease progression. J Bone Miner Res. 2014; 29(1):55–66. PMID: 23787729.

10. Ogino K, Ogura K, Kinugasa Y, Furuse Y, Uchida K, Shimoyama M, et al. Parathyroid hormone-related protein is produced in the myocardium and increased in patients with congestive heart failure. J Clin Endocrinol Metab. 2002; 87(10):4722–4727. PMID: 12364464.

Fig. 1
Clinical photograph of the patient. (A) Macroglossia. (B) Generalized edema predominantly seen in lower limb (The figures are published under agreement of the patient with a second informed consent).
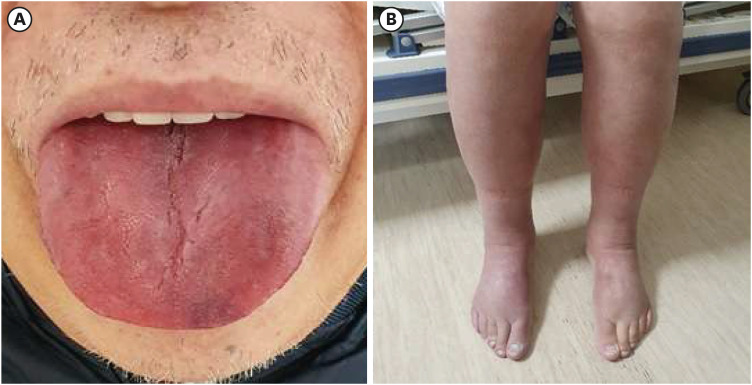
Fig. 2
Initial findings of the patients. (A) Initial electrocardiogram presented non-specific conduction delay. (B) Chest X-ray showed cardiomegaly with pleural effusion (The figures are published under agreement of the patient with a second informed consent).
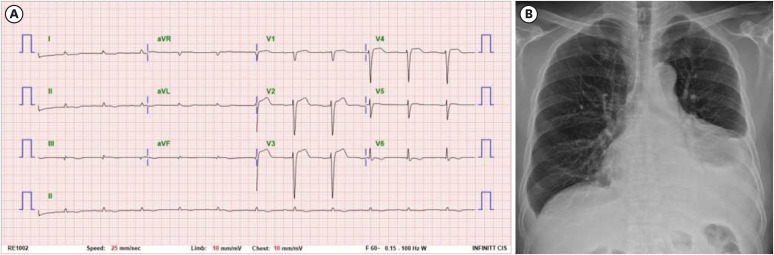
Fig. 3
Echocardiogram showed normal systolic function and no regional wall motion abnormality in parasternal long-axis view (A), and apical four-chamber view (B). Moderate left ventricular hypertrophy (left ventricular wall thickness = 1.6 cm) was obvious. Pulsed wave Doppler recording across the mitral valve showed diastolic dysfunction with restrictive pattern (C). Tissue Doppler image (D) and strain Doppler image (E) presented the relaxation abnormality with global strain reduction.
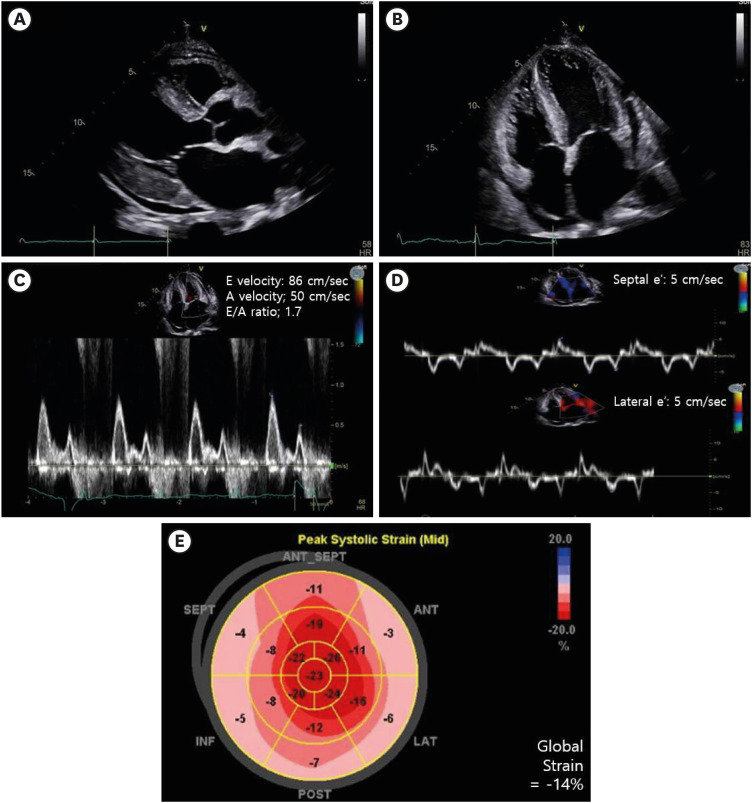
Fig. 4
Tissue biopsy. (A) Tongue biopsy showed subepithelial amorphous material depositions (H&E ×200). (B) Congo Red staining under a polarizing microscope showed apple green birefringence on the tongue, consistent with amyloid staining (×200). (C) Endocardial biopsy showed intramuscular amorphous material deposition (H&E ×200). (D) Congo Red staining under a polarizing microscope shewed apple green birefringence on the endocardium (×200).
H&E = hematoxylin and eosin.
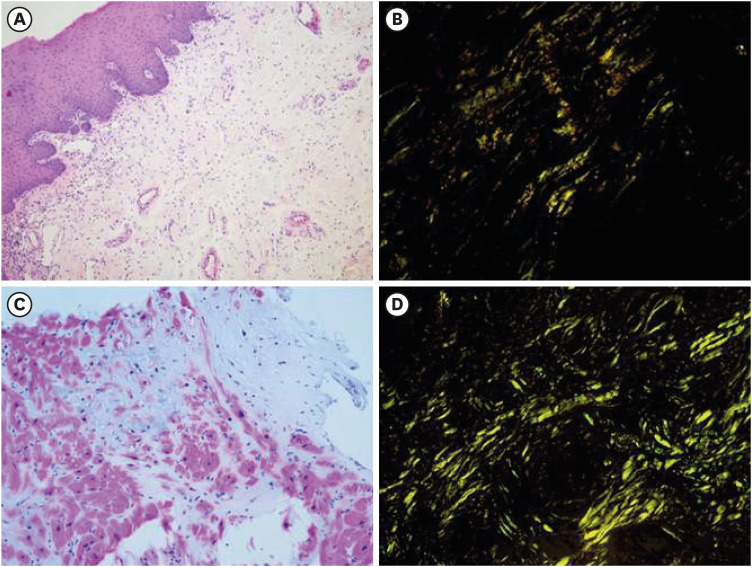
Fig. 5
Bone marrow biopsy. (A) CD138 plasma cell immunohistochemical staining showed 10% plasma cell cellularity (×200). (B) Bone marrow was positive for lambda immunohistochemical staining (×400).
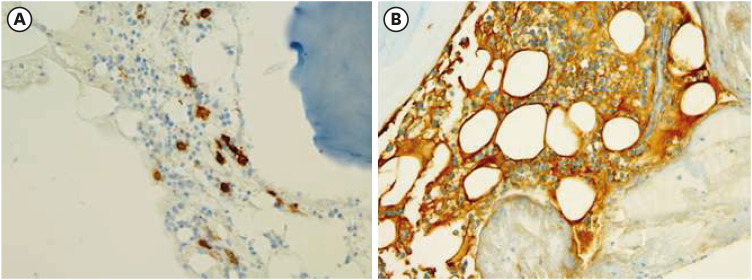
Table 1
Initial blood test results related to calcium metabolism
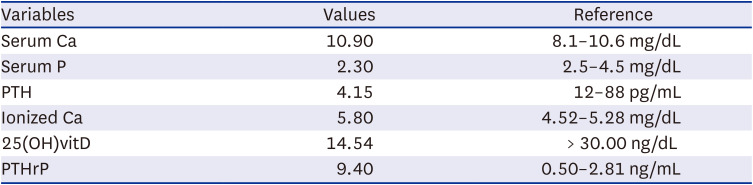
| Variables | Values | Reference |
|---|---|---|
| Serum Ca | 10.90 | 8.1–10.6 mg/dL |
| Serum P | 2.30 | 2.5–4.5 mg/dL |
| PTH | 4.15 | 12–88 pg/mL |
| Ionized Ca | 5.80 | 4.52–5.28 mg/dL |
| 25(OH)vitD | 14.54 | > 30.00 ng/dL |
| PTHrP | 9.40 | 0.50–2.81 ng/mL |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download