INTRODUCTION
Coronavirus disease 2019 (COVID-19) emerged in China in 2019 and rapidly spread worldwide, resulting in a global pandemic.
1 According to the World Health Organization (WHO), as of November 2022, over 600 million confirmed cases of COVID-19 and over 6.5 million deaths had been reported.
2
Remdesivir is the first and only antiviral agent approved by the US Food and Drug Administration (FDA) to treat COVID-19.
1 It acts as an RNA-dependent polymerase inhibitor of viral replication. Initially, remdesivir was approved for use in patients with severe COVID-19, but its indication has been expanded for use in high-risk patients who are expected to progress to severe disease.
345 Clinical trials, including the Adaptive Covid-19 Treatment Trial (ACTT-1), have demonstrated that remdesivir improves the recovery rate and is well tolerated in the treatment of COVID-19.
6789 Network meta-analyses of clinical trials have also shown that remdesivir treatment is not significantly associated with increased risk of adverse events (AEs) and reduces serious AEs (SAEs) which include septic shock, cardiac arrest, respiratory failure, mechanical ventilation, pulmonary embolism, deep venous thrombosis.
1011
However, data from the global pharmacovigilance database, WHO VigiBase®, suggest that remdesivir is associated with hepatic enzyme increases, renal injury, and creatinine level rises, indicating a need to monitor patients for these AEs.
12 Additionally, case reports, case series, and randomized control trials (RCTs) suggest that AEs such as bradycardia, hepatotoxicity, nausea, diarrhea, hypokalemia, headache, respiratory toxicity, nephrotoxicity and reproductive toxicity may occur with remdesivir treatment.
1314
Clinical trials are conducted under strictly controlled situations for a selected population, and their reported AEs may not fully reflect the situation in real-world patients, including vulnerable populations such as those with renal or hepatic insufficiency or pregnant women. The approved product label of remdesivir does not recommend its use in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min due to the absence of an assessment and concerns over the accumulation of sulfobutyl-ether beta-cyclodextrin-sodium excipients.
315 Additionally, initiating remdesivir in patients with alanine aminotransferase (ALT) ≥ 5 × the upper limit of normal at baseline is not recommended by the European Medicines Agency (EMA) and early Korean guidelines.
1516 According to FDA and the latest Korean guidelines, remdesivir should be discontinued if ALT levels exceed ten times the upper limit of normal.
317
Despite these recommendations, remdesivir is sometimes used in vulnerable populations in real-world clinical practice. Multicenter case series in the United States have described changes in ALT and serum creatinine during remdesivir therapy and reported AEs in hospitalized COVID-19 patients with an eGFR < 30 mL/min per 1.73 m
2 or receiving renal replacement.
18 In addition, a retrospective study conducted at a hospital in New York found that remdesivir was not associated with acute kidney injury (AKI) or hepatotoxicity in patients with estimated creatinine clearance (eCrCl) < 30 mL/min compared to those with eCrCl ≥ 30 mL/min.
19 However, safety data on remdesivir in real-world clinical situations, particularly in vulnerable populations, are insufficient. Few studies have investigated the safety of remdesivir in renal impairment patients. To our knowledge, there are no studies on AEs caused by remdesivir in patients with hepatic impairment except for some case reports.
20
Therefore, we aimed to estimate the incidence of adverse drug events (ADEs) during remdesivir treatment through a multicenter medical record review with causality assessment in the overall population and in vulnerable subpopulations such as those with impaired renal or hepatic function and pregnant women.
METHODS
Study design and study population
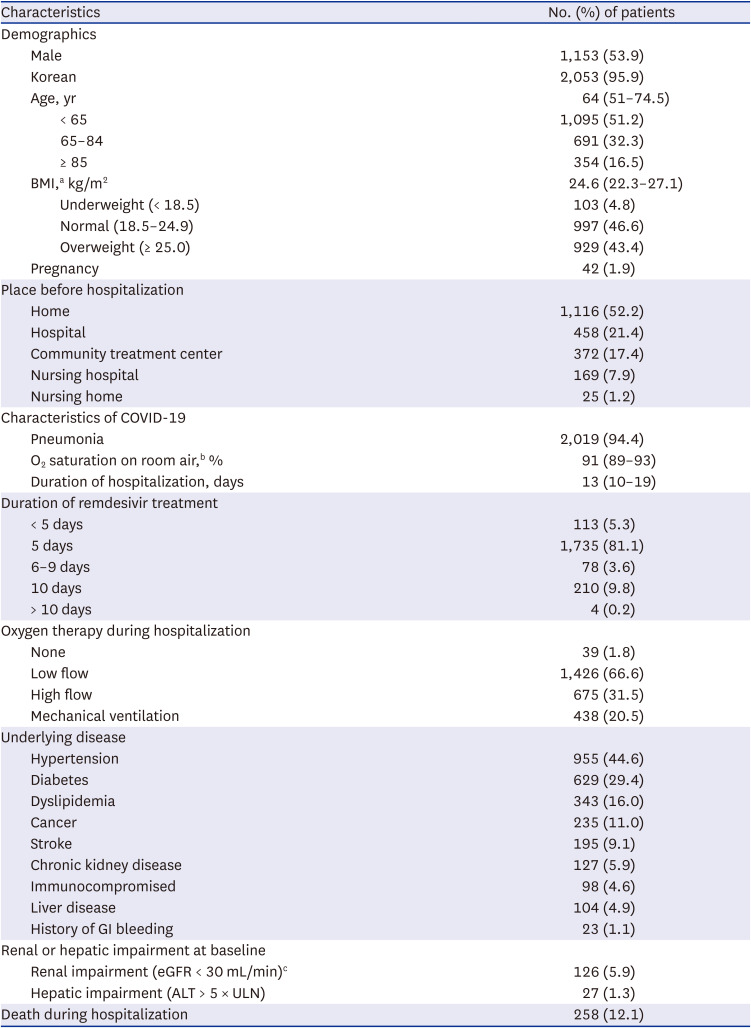
This retrospective observational study was conducted at eight tertiary and two general hospitals in Korea (
Supplementary Table 1). The study population consisted of hospitalized patients with confirmed COVID-19 who received remdesivir between January and December 2021. Patients were excluded if they did not undergo blood laboratory tests before starting remdesivir or if they were transferred from another hospital after commencing treatment with remdesivir. To avoid hospital bias, we enrolled a maximum of 300 patients from each hospital, and for hospitals with more than 300 eligible patients, 300 patients were randomly selected for enrolment. Information was gathered independently by trained pharmacists and researchers from 10 hospitals, and when there was disagreement or uncertain decision, two people discussed and decided.
For subpopulation analysis, we defined patients with renal impairment as those with pre-treatment eGFR < 30 mL/min and patients with hepatic impairment as those with an ALT value > 5 times the upper limit of normal at baseline. We calculated the eGFR using the Modification of Diet in Renal Disease equation and body surface area. In patients for whom eGFR could not be calculated, we used the creatinine clearance (CrCl) calculated using the Cockcroft and Gault equation. Pregnant women were identified with medical records.
Definition of ADEs
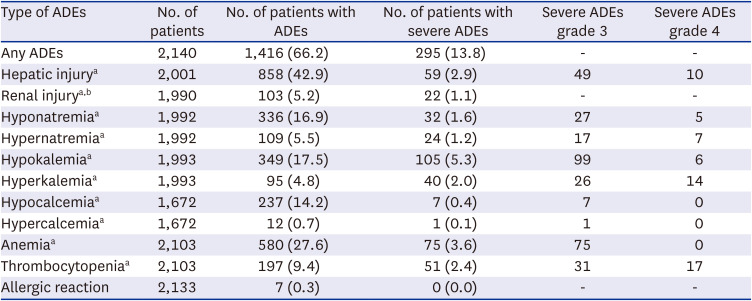
ADEs were defined as hepatotoxicity, nephrotoxicity, electrolyte abnormalities, anemia, thrombocytopenia, and allergic reactions requiring treatment. These ADEs were chosen because they could be detected objectively to minimize any biases that may arise from deviations in the medical records, considering the nature of retrospective and multicenter studies. ADEs except ‘renal injury’ were defined based on the National Cancer Institute’s grading system of Common Toxicity Criteria for Adverse Events (NCI-CTCAE) and NCI-CTCAE grade ≥ 3 was used to define the severe ADE. ‘Renal injury’ was defined based on the Kidney Disease: Improving Global Outcomes criteria (
Supplementary Table 2). The causality of all ADEs was assessed with the WHO-Uppsala Monitoring Centre (WHO-UMC) tool, and cases assessed as ‘possible’, ‘probable’, or ‘certain’ were considered as ADEs.
We evaluated ADEs that occurred from the start date of remdesivir treatment to the 7th day of treatment end, taking into account the half-life of the remdesivir metabolite (approximately 27 hours)
21 or discharge date or death, whichever came first. Patients without baseline laboratory results were excluded from the evaluation of individual ADEs, and patients with dialysis before using remdesivir were excluded in calculating the incidence of ‘renal injury.’
Statistical analysis
The incidences of ADEs and severe ADEs were calculated as the number of patients who experienced at least one ADE divided by the total number of patients. The incidence of individual ADEs was estimated.
For the subpopulation analysis, we constructed a 1:4 matched control group for each subpopulation with a propensity score. Propensity scores were estimated using the following variables: sex, age, body mass index (BMI), oxygen therapy, mechanical ventilation, hypertension, diabetes, dyslipidemia, stroke, immunocompromised status, malignancy, and duration of remdesivir use. The incidence of ADEs in each subpopulation and the corresponding controls were calculated after matching.
Descriptive statistics were expressed as median (interquartile range [IQR]), mean ± standard deviation, or frequency with percentage. All statistical analyses were performed using SAS enterprise guide 8.3 (©2019–2020, SAS Institute Inc., Cary, NC, USA).
Ethics statement
The study was reviewed and approved by the Institutional Review Board of each hospital (approval No. H-2203-084-1308, 10-2022-45, B-2204-753-404, 2022-05-047, 2022-03-028, 2022-04-030, 2203-032-113, 2022-03-046, 2022-03-025, and CNUH-2022-104). Informed consent was waived because of the retrospective nature of the study.
DISCUSSION
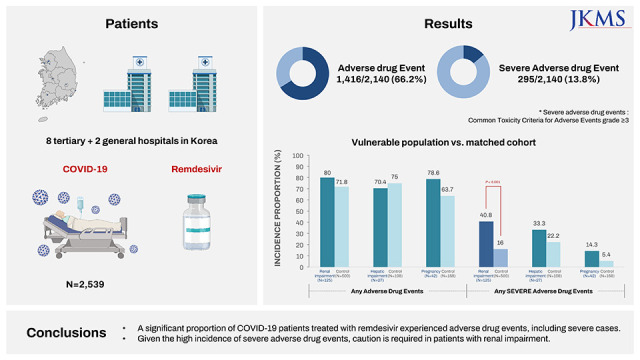
In this study, 66.2% of COVID-19 patients treated with remdesivir experienced at least one ADE, while 13.8% experienced a severe ADE. These findings align with previous results. In a multi-country RCT involving 199 patients on 5-day remdesivir therapy and 197 patients on 10-day therapy across 105 hospitals in the United States, Europe, and Asia, the remdesivir group (both 5-day and 10-day groups) exhibited any AEs of 51–59%, severe AEs (grade 3 or higher) of 10–12%, and SAEs of 5%. The incidence of laboratory abnormalities was 72–73% for any grade, 10–14% for grade 3, and 2–3% for grade 4.
9 However, the incidence of ADEs in our study differed from those reported in other studies. In a representative trial called ACTT-1, the remdesivir group exhibited lower rates of at least one AEs (57.3%), SAEs (24.6%), and grade 3 or 4 AEs (51.3%) compared to the placebo group.
6 Another RCT conducted in China analyzed 158 patients treated with remdesivir and observed AEs of 65.8%, SAEs of 18.1%, and severe AEs (grade 3 or 4) of 8.4%.
7 In a study involving 397 confirmed COVID-19 patients who did not have a placebo group like ours, the 5-day and 10-day remdesivir groups reported AEs of 70–74%, SAEs of 21–35%, and grade ≥ 3 laboratory abnormalities of 27–34%.
8 These variations can be attributed to methodological differences, AEs definitions, study design, and population characteristics. The AEs reported in these studies included clinical situations related to the progression of COVID-19, such as respiratory failure and hypoxia. In contrast, our study focused solely on ADEs that could be determined through laboratory investigations. Additionally, previous studies excluded patients with hepatic impairment, renal impairment, and pregnant women, whereas the observation period in our study varied from that of the referenced studies.
The major ADEs identified in this study were hepatic injury (42.9%) and anemia (27.6%); the most frequent severe ADE was hypokalemia (5.3%). Similar AEs such as ‘hepatic injury’, ‘total bilirubin increase’ and ‘AST increase,’ ‘anemia,’ ‘decreased GFR,’ and ‘decreased hemoglobin level’ were also reported in the previous studies.
6789 In addition, several AEs such as nausea, vomiting, rash, and bradycardia that were not investigated in our study are presented as common AEs in other studies.
622
However, Electrolyte imbalances such as hypokalemia, hyponatremia, and hypocalcemia were observed in more than 10% of the patients in our study, which is not included in the current label of remdesivir.
315 Additionally, hypokalemia was reported in some RCTs
79 but other electrolyte imbalances observed in this study have not been reported.
Anemia has been reported in 7.9% and 12% of patients in some studies, which were lower than our findings.
67 In an Italian observational study, the anemia was higher in COVID-19 patients than in the control group.
23 Rapid hydration, a symptomatic treatment of COVID-19,
1 may have contributed to decreased hemoglobin concentrations by a diluting effect, which was captured as anemia in this study.
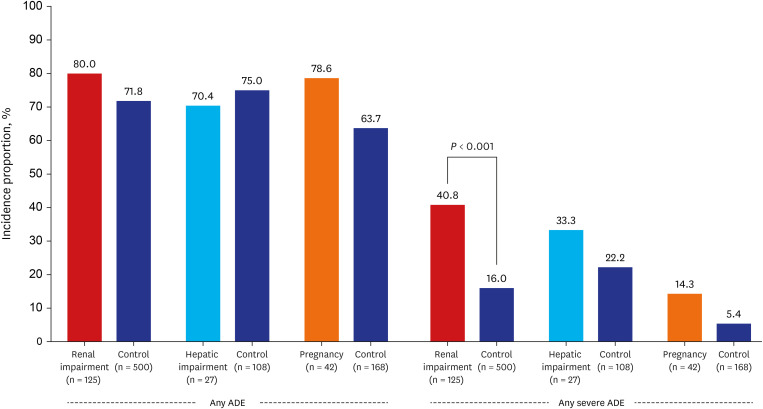
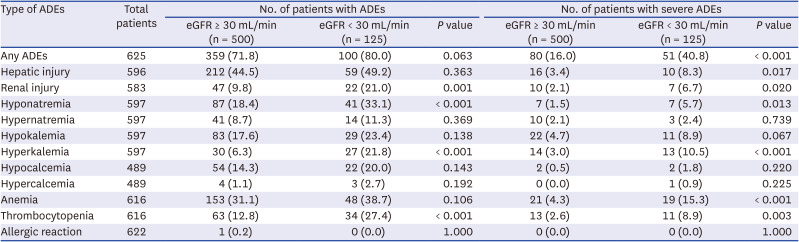
Our study showed no difference in the incidence of ADEs between 126 patients with renal impairment at baseline and matched controls. Nonetheless, severe ADEs were higher in patients with renal impairment. This finding is somewhat different from those of previous studies. In a study of 416 patients in New York, 55 patients had renal impairment with CrCl (eCrCl) < 30 mL/min, and there was no significant difference in the incidence of AKI and hepatotoxicity compared to those without.
19 In this study, the definitions of renal injury and hepatic injury were different from our study, so there may be differences in the results. In addition, a study investigating 46 patients with renal impairment using remdesivir in India concluded that remdesivir was well tolerated by patients with AKI or chronic kidney disease.
24 However, patients with chronic kidney disease can have poor outcomes of COVID-19,
25 which may have contributed to our high incidence of severe ADEs. considering that the metabolite of remdesivir or sulfobutyl-ether beta-cyclodextrin-sodium carrier can accumulate in renally impaired patients, and the incidence proportion of severe ADEs was higher in patients with renal impairment in our study, caution is needed when using remdesivir in patients with renal impairment.
In this study, there was no statistically significant difference in the incidence of ADEs and severe ADEs between the patients with and without hepatic impairment. While EMA and early Korean guidelines recommend that remdesivir should not be started if ALT exceeds five times the upper limit of normal,
1516 FDA approvals and the latest Korean guidelines do not restrict the use of remdesivir according to baseline ALT levels.
317 Considering these inconsistencies and the fact that our study analyzed a small number of 27 patients with hepatic impairment at baseline, further studies are needed in patients with hepatic impairment.
Additionally, this study found no significant difference in the incidence of ADEs and severe ADEs between pregnant and non-pregnant patients, which confirmed the findings from previous studies that the use of remdesivir by pregnant patients had a high recovery rate and was well-tolerated.
26 However, considering a trend toward a higher incidence of severe ADE in pregnant women, further studies are needed.
Our study had some limitations. First, due to the retrospective design and operationally defined ADEs with objective findings, there is a possibility of underestimating the incidence of ADEs. Gastrointestinal problems, such as nausea and vomiting, which are commonly mentioned AEs with remdesivir use, as well as the potential ADEs not defined in this study, such as bradycardia and hyperglycemia, were not collected. Second, as there was a lack of a control group that did not use remdesivir and most of the causality assessment results for ADEs were ‘possible,’ it is difficult to distinguish whether ADEs were caused by remdesivir, COVID-19, or underlying disease. Third, although we tried to obtain generalized data through a multicenter study in Korea, the study findings might not be generalizable to patients of other races or countries. Fourth, since this study targeted patients with severe COVID-19, it may be difficult to apply to patients with mild COVID-19 who are at risk of progressing to severe disease, which is an expanded indication. Further studies with these patients are required. Lastly, we did not take into account the stage of pregnancy during the exposure to remdesivir. The gestational age of the pregnant woman has been known to affect adverse drug reactions and even the severity of COVID-19 infection.
27 It is crucial for future studies to consider this factor when assessing the impact of pregnancy on the ADEs of remdesivir.
However, this study provides important insights into the safety profile of remdesivir in real-world settings, including vulnerable subpopulations. It was meaningful because it did not simply detect AEs according to changes in laboratory results but used a causality assessment tool to confirm causality with remdesivir.
In conclusion, while the limitations of a retrospective analysis without a control group prevented us from assessing treatment-relatedness, this study revealed that approximately two-thirds and more than one-eighth of patients with COVID-19 experienced at least one ADE and severe ADE, respectively, during treatment with remdesivir. There was no difference in the incidence of ADEs in the subpopulations with renal impairment, hepatic impairment, or pregnancy compared to those without. However, it is important to note that patients with renal impairment exhibited a significantly higher incidence of severe ADEs compared to those without such impairment. As a result, the use of remdesivir in a particular population is not recommended unless the potential benefits clearly outweigh the associated risks.
317 In cases where remdesivir is administered to patients with renal impairment, it is crucial to closely monitor the occurrence of ADEs and promptly initiate appropriate laboratory test or treatments as needed.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download