DISCUSSION
In this study, the etiologic distribution and clinical characteristics of CAP in Korean children with or without UMC for 5 years before the COVID-19 pandemic were investigated through an intensive selection procedure for evident pneumonia and strict criteria for the positive results of the etiologic tests. As a result, the detection rate of M. pneumoniae was the highest, followed by RSV, and the incidence of pyogenic bacterial CAP was very low at 2.3%. Nevertheless, most and half of hospitalized children with CAP received parenteral β-lactam and oral macrolide antibiotics, respectively. In addition, even though life-supporting management such as mechanical ventilation and ICU care and poor outcomes such as PIBO and mortality were mostly encountered among children with underlying medical conditions, parenteral antibiotics were used for previously healthy children with CAP with a similar frequency.
Pneumonia is known to be one of the most common causes of hospitalization among children. The main reasons for hospitalization might be the need for close monitoring of respiratory distress and secondary complications, respiratory support, and parenteral antibiotic treatment. The diagnosis of pneumonia is also a common reason for the use of oral antibiotics in the outpatient clinic. However, in many studies, even those from developed countries, a substantial proportion of CAP is caused by respiratory viruses, whereas the proportion of pyogenic bacterial pneumonia is relatively small.
214 Nevertheless, empirical β-lactam antibiotics are widely used due to the limitations in diagnosing the causative bacteria of pneumonia in clinical practice.
15
Accurate clinical diagnosis of pneumonia is not always easy. Of course, if there are clear CXR findings indicative of pneumonia, such as lobar consolidation and a moderate amount of PE, with acute onset of respiratory symptoms and abnormal auscultation, pneumonia should be evident, and even the possibility of a pyogenic bacterial etiology might be high. However, in many clinical situations, CXR shows nonspecific findings, such as peribronchial or parahilar infiltrations on the nonfully inspired lungs of infants and toddlers. In addition, rales/crackles, which are known to be an indicative auscultation finding of pneumonia, could also be accompanied by viral bronchiolitis. These limitations cause pneumonia to be overdiagnosed and thus antibiotics to be overused. Furthermore, in many clinical studies conducted without this distinction being clear, the proportion of viruses as the etiology of pneumonia may appear larger than in reality. In the current study, as a result of applying very strict pneumonia diagnosis criteria, only 18% of cases with a pneumonia diagnosis and/or CXR findings of pneumonic infiltrations were selected among all clinically diagnosed pneumonia cases. It is assumed that a large number of acute respiratory tract infections have been misdiagnosed as pneumonia and thus resulted in hospitalization.
Additionally, in the etiologic diagnosis of pneumonia, many special considerations are required for the appropriate interpretation of radiographic and laboratory tests. Since it is almost impossible to collect samples from the lung parenchyma itself, the specific pathogen is assumed to be present primarily based on clinical features and CXR findings in usual clinical situations or by some NPA/NPS or sputum tests. However, infants and children are not able to spit out sputum properly. As a result, the etiologic diagnosis of pneumonia is mainly performed with mRT-qPCR tests from NPA/NPS for respiratory viruses, PCR tests from NPA/NPS or serological tests for atypical bacteria, and conventional culture from blood, PE, BAL fluid or some endotracheal aspirates (ETAs) for pyogenic bacteria. Additional PCR tests, including rRNA sequencing for atypical and pyogenic bacteria and antigen testing for
S. pneumoniae both from PE or BAL fluid, could also be utilized.
15
However, URT specimens may not properly represent the etiology of LRTIs. For example,
S. pneumoniae, the most common cause of pyogenic bacterial pneumonia, commonly colonizes in infants and young children and can be detected at any time even in healthy children without symptoms. This frequent colonization of
S. pneumoniae could also induce positivity in the blood PCR and urinary antigen tests, which can be used for adults. On the other hand, when the amount of blood for culture is insufficient or antibiotics are administered before culture, the yield of the blood culture could decrease.
16 Moreover, some respiratory viruses, such as HRV, HCoV, and HBoV, could also be detected in asymptomatic children due to their prolonged shedding and recurrent asymptomatic/mild symptomatic infections as well as the false positivity of PCR tests. Additionally,
M. pneumoniae can be PCR positive from NPS/NPA specimens of asymptomatic children depending on the epidemic period and country,
17 and serological tests have even more limitations. Two blood samplings in both acute and convalescent periods are not practical, and the immunoglobulin M (IgM) test is known to be positive for a very long time and thus cannot reliably differentiate acute and previous infection.
1718
The proportions of CAP etiologies among children have been widely reported depending on country, period, and age group.
2141920 This also largely depends on which tests are used for the identification of each causative organism and at what level the results are accepted as positive. To determine the more accurate distribution of CAP etiologies among Korean children, only respiratory viruses known to be meaningful from NPS/NPA specimens were included, and PCR detection and serological test results of at least a 4-fold increase or a higher single antibody titer corresponding to 1:640 of IAT were considered for
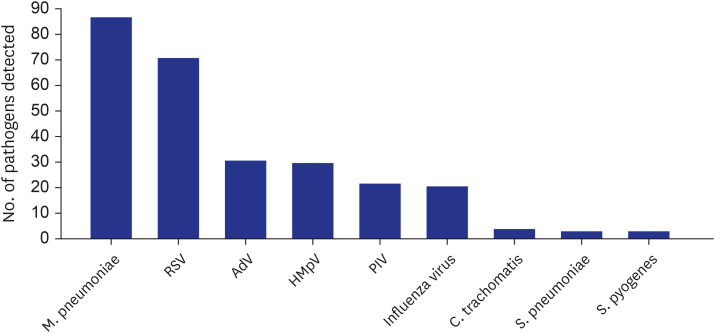
M. pneumoniae in the current study. For pyogenic bacteria, the possibility of detection of colonizers was aggressively excluded. As a result, more than one pathogen was detected in only half of CAP cases, comprising respiratory viruses in 32.3%, atypical bacteria in 17.8%, and pyogenic bacteria in 2.3%. These proportions were much lower than those of recent etiologic studies in the US, except for
M. pneumoniae.
214 In these multicenter prospective studies, any pathogen was detected in 65–81% of patients, including viruses in 56–73%, atypical bacteria in 8–9%, and pyogenic bacteria in 4–7%. This might be partly because they used more rigorous etiologic workups, including serologic tests for viruses and/or blood PCR for pyogenic bacteria. On the other hand, recent retrospective and prospective multicenter etiologic studies in South Korea reported a much higher proportion of
M. pneumoniae at 29.6% and 41.3%, respectively.
56 This is probably due to the low specificity of serologic tests for
M. pneumoniae.
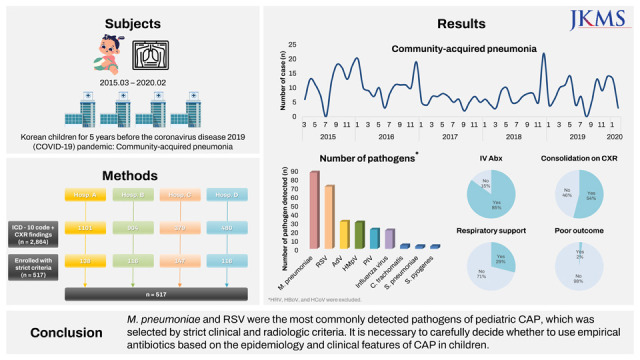
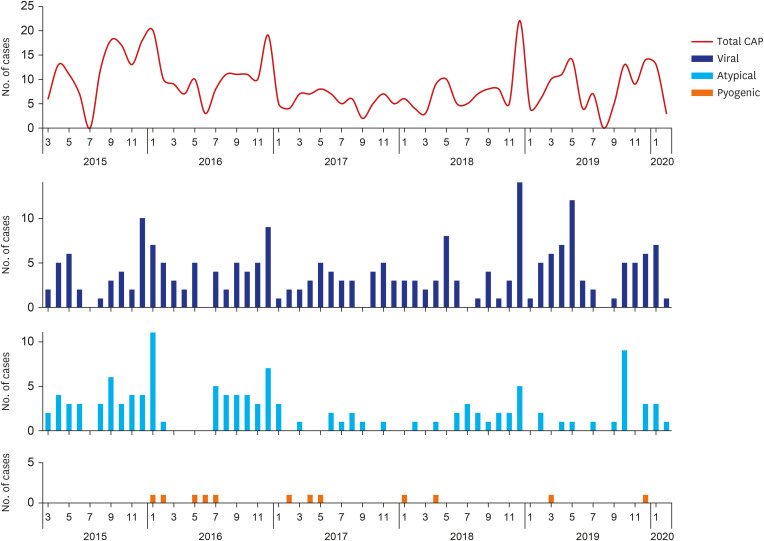
The number of children hospitalized with CAP and the detection frequency of both respiratory viruses and atypical bacteria were highest in the winter season during the current study period. This shows that RSV and
M. pneumoniae have a large effect on hospitalization for CAP among Korean children with or without UMCs during the epidemic period almost every year.
2122 Moreover, there was also an annual increase in the number of CAP hospitalizations in March–May and September–October, which are early school opening periods, and the lowest annual prevalence of CAP admissions during the summer vacation season. This suggests that the circulation of respiratory pathogens in schools also largely affects the occurrence of CAP among children. In this regard, large changes in the epidemiology of CAP in children might have occurred during the COVID-19 pandemic and even thereafter. We should note these changes to properly respond to new CAP epidemics among children.
In addition to RSV, other respiratory viruses, such as AdV, HMpV, PIV, and Flu, were commonly detected and induced the year-round prevalence of CAP among children. Regarding atypical bacteria, it is noteworthy that the proportion of C. trachomatis was relatively high. Because diagnostic PCR testing for C. trachomatis was performed only in the selected cases and hospitals, there might be more cases where a diagnosis was not made and improved by the use of empirical macrolide antibiotics. It is also noteworthy that the detection frequency of S. pyogenes was as high as that of S. pneumoniae among pyogenic bacteria. The influence of H. influenzae, which has been classically thought to be a major bacterial etiology, or S. aureus, which has recently been detected frequently in CAP, did not appear in the current study.
Toddlers aged 1–4 years and children aged 5–11 years comprised more than three quarters (76.6%) of all children hospitalized with CAP during the study period. The frequencies of dyspnea (19.9%) as an initial symptom and CXR findings of consolidation (54.4%) and PE (13.5%) were relatively low considering that all CAP cases were hospitalized in a referral hospital. Moreover, these proportions were even lower among previously healthy children. However, most (85.1%) patients received parenteral β-lactam antibiotics on admission for CAP, and this proportion was comparable between children with and without UMCs. Considering the detection rates of pyogenic bacteria (2.3% in overall and 1.1% in healthy children) in the current study, the rate of antibiotic use was too high even considering the low sensitivity of the test for bacteria. Moreover, this management strategy has probably resulted in unnecessary hospitalizations for relatively mild CAP that probably has a viral or mycoplasma etiology.
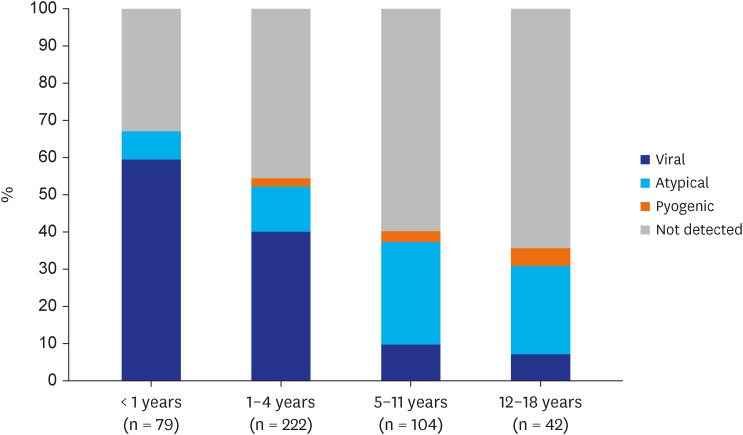
In the current study, 6 of 10 children aged 5–18 years with CAP had no pathogen detected. This was probably because very strict criteria for the serologic test were used, and the PCR test was not uniformly performed for atypical bacterial pathogens, particularly M. pneumoniae. Some pyogenic bacterial pathogens may have been missed due to the low sensitivity of their etiologic detection. However, an unknown pathogen not routinely tested for could be an etiology for CAP in school-aged children and adolescents. Vigorous etiologic investigation with novel technologies is warranted for CAP in this age group.
The current study aimed to properly investigate the epidemiology and clinical characteristics of CAP in children with or without UMCs by using strict guidelines with up-to-date evidence for etiologic diagnosis. Nevertheless, this study had several limitations. First, since this was a retrospective study, the adequacy of CAP diagnosis could not be perfect even if strict criteria were applied. In addition, etiologic workups were not uniformly performed at each study site. Thus, it is possible that a number of pathogens were missed, and the effect of superinfections may have been underestimated. Furthermore, pyogenic bacterial infection may be underdetected due to the previously mentioned endogenous limitations of pneumonia etiology studies among children. It is also possible that the rates of viral and atypical bacterial CAP were also underestimated due to the exclusion of the possibly causative respiratory viruses HRV, HBoV, and HCoV, and the much higher cutoff level for the positive single serologic test titer for M. pneumoniae. In addition, this study did not include healthy controls for the detection rate of respiratory pathogens, so we could not confirm the causality of each pathogen detected in children with CAP. PIBO was not uniformly evaluated and captured for all patients; thus, the prognosis of CAP, particularly in previously healthy children, could have been underestimated. However, because we considered only PIBO with clinically notable respiratory symptoms and signs, those with these clinical features were probably evaluated with a CT scan. Finally, although this was a multicenter study, the results of this study may have been biased due to the large heterogeneity of each institution and the geographical location. Therefore, there is still a limitation in generalizing the results of this study to all institutions. Nevertheless, the proportion of a viral etiology was high even among children hospitalized in referral hospitals, and the actual rate of CAP seems to be exaggerated. The greatest importance of the results from this study is that many children with CAP can be treated without parenteral antibiotics in an outpatient setting.
In conclusion, more stringent criteria should be applied to detect actual pediatric pneumonia cases and then properly manage them. If possible, sufficient etiologic workup is recommended, and the results should be appropriately interpreted with caution. Previously healthy Korean children with CAP might initially be treated without hospitalization for parenteral antibiotic treatment unless there is a considerable clue for a pyogenic bacterial etiology, such as a very high concentration of inflammatory markers and lobar/lobular consolidation on CXR.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download