INTRODUCTION
1) Orthophosphoric acid (OPA) (Scotchbond Universal Etchant, 3M ESPE; St. Paul, MN, USA) and amla juice as an acid etchant (groups 1 and 3)
2) CHX (Consepsis; Ultradent Inc., South Jordan, UT, USA) and amla extract as MMP inhibitors with OPA as an etchant (groups 2 and 4)
3) OPA etchant with CHX as an MMP inhibitor (group 2) and amla juice etchant with amla extract as an MMP inhibitor (group 5)
4) Amla etchant as an MMP inhibitor (group 5) and amla etchant or amla MMP inhibitor (group 3 or 4)
MATERIALS AND METHODS
Extraction and preparation of pure amla juice and amla extract
SEM study for assessing the etching effect on dentin
Gelatin zymography to assess MMP activity
• Untreated mineralized dentin powder served as a negative control.
• Dentin powder demineralized with 1% OPA for 10 minutes at 4°C served as the positive control.
• Dentin powder demineralized with 1% OPA for 10 minutes at 4°C and then incubated with 50% and 100% amla extract for 30 minutes served as the study groups.
1) Dentin protein extraction: 25 mg of the amla-treated sample amla was mixed with 300 μL of ice-cold lysis buffer, ground with a mortar, and maintained in constant agitation at 4ºC for 2 hours. It was then centrifuged for 20 minutes at 12,000 rpm at 4°C in a micro-centrifuge. The tubes were gently removed from the centrifuge and the supernatant was subjected to total protein estimation using the Bradford assay. Samples were prepared in a standard non-reducing loading buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis with the gelatin substrate embedded in the separating gel.
2) Zymographic analysis: Following electrophoresis, sodium dodecyl sulfate was removed from the gel by incubation in un-buffered Triton X-100. The gel was rinsed for 5–10 minutes in the incubation buffer at 37°C with agitation. A fresh incubation buffer was added and incubated for 24 hours at 37°C. The gel was stained using Coomassie Brilliant Blue staining solution (Brilliant Blue R Concentrate; Sigma-Aldrich) for 30 minutes to 1 hour and finally rinsed with water to remove the excess staining solution. The gel was incubated with a destaining solution until the bands were visible.
3) Setting the effective concentration of amla extract with MMP activity: Areas of enzyme (MMPs) activity appeared as white bands against a dark blue background band. The concentration at which the amla extract (either 50% or 100%) showed an effective decrease in the intensity and thickness of the white band was considered the effective concentration of amla extract as an MMP inhibitor and used in further procedures.
SEM study for assessing structural changes caused by an MMP inhibitor on dentin
Microshear bond strength of composite resin
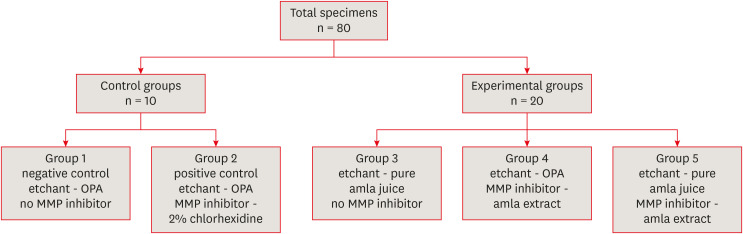
1) Specimen preparation: The roots of 80 selected teeth were cut 2 mm below the cemento-enamel junction and the occlusal surfaces were ground to expose the dentin in order to obtain dentinal slabs with a uniform thickness of 3 mm. The exposed dentinal surfaces were finished using 600-grit silicon-carbide papers to create a standardized smear layer. Slabs obtained were then mounted in cold cure acrylic resin (DPI-RR Cold cure; Dental Products India, Mumbai, India) using square rubber molds of standardized dimensions (25 × 25 mm). The 80 prepared specimens were then assigned into control and experimental groups, as shown in Figure 1. To standardize, acid etchants (either 37% OPA or pure amla juice) were applied on the dentin surface for 15 seconds using applicator tips, rinsed with water spray for 10 seconds, and then air-dried for 5 seconds. MMP inhibitors (either 2% CHX or 100% amla extract) were applied for 60 seconds using applicator tips, washed off, and air-dried. After preparing the samples according to the control and experimental groups, the bonding protocol described below was followed.
2) Bonding protocol: For all specimens, irrespective of their groups, a dentin adhesive (3M Single bond Universal adhesive; 3M ESPE) was gently rubbed using an applicator tip for 20 seconds, followed by gentle air-drying for 5 seconds and light-curing using a light-emitting diode light-curing unit (Woodpecker Med. Instrument; Guilin, China) for 10 seconds. Polyethylene tubes with an internal diameter of 2 mm and a height of 2 mm were firmly attached over the bonded surface of the dentin to expose a standardized dentinal surface area with a diameter of 2 mm. Nanocomposite resin (C1 body shade of 3M Filtek™ Z350XT universal restorative; St. Paul, MN, USA) was placed inside the polyethylene tube in 2 increments of 1 mm thickness, each increment was cured for 20 seconds at a radiant emittance of 400 mW/cm2 at zero distance. The tubes were then removed with a sharp knife after composite build-up and were stored in distilled water for 2 weeks at 37°C.
3) Bond strength evaluation procedure: After being stored in distilled water, each acrylic resin block along with its bonded cylinders was fixed to the lower compartment of a universal testing machine (TEC-SOL India; Chennai, India) using tightening screws. A loop of orthodontic stainless steel wire (0.2 mm in diameter) was wrapped around the bonded cylinder as close to its base as possible and aligned with the load cell axis of the upper movable compartment of the testing machine. The specimens were then subjected to microshear bond strength testing with a load cell of 5 kN at a crosshead speed of 0.5 mm/min at the dentin-composite resin interface until fracture occurred, and the data were recorded using a computer software (TEC-SOL India). The operator performing the bond strength tests was blinded during the entire procedure. The microshear bond strength values (MPa) were calculated using the following formula: microshear bond strength = peak load at failure/bonded surface area.
Statistical analysis
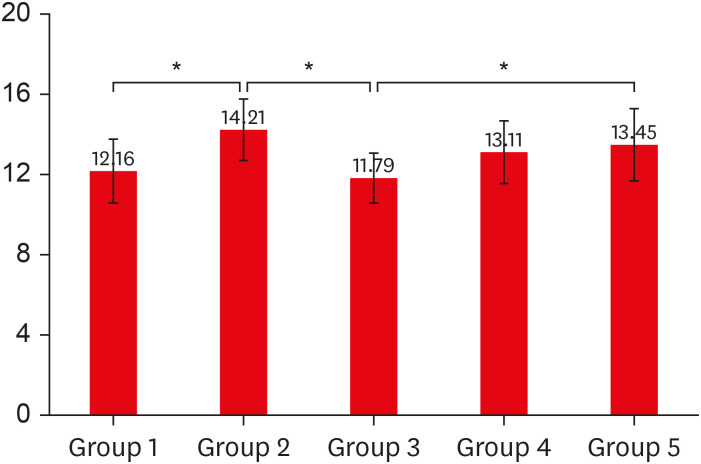
RESULTS
Comparison of the etching effect of amla juice with 37% OPA on dentin by SEM
Effective concentration at which amla extract acts as an MMP inhibitor by gelatin zymography
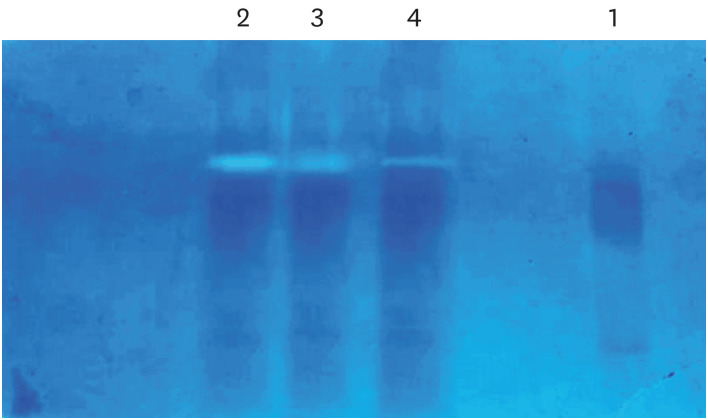 | Figure 3Effective concentration at which amla extract acts as an matrix metalloproteinase inhibitor as determined using gelatin zymography.Lane 1, untreated mineralized dentin powder (negative control); Lane 2, dentin powder demineralized with 1% OPA for 10 minutes at 4ºC (positive control); Lane 3, dentin powder demineralized with 1% OPA for 10 minutes at 4ºC and then incubated with 50% amla extract for 30 minutes; Lane 4, dentin powder demineralized with 1% OPA for 10 minutes at 4ºC and then incubated with 100% amla extract for 30 minutes.
OPA, orthophosphoric acid.
|
Comparison of the effect of amla extract with 2% CHX as MMP inhibitors on dentin by SEM
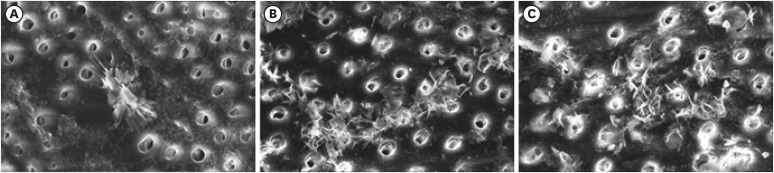 | Figure 4Scanning electron microscopic images evaluating and comparing different etchants and MMP inhibitors at ×5,000 magnification. (A) Etched with 37% OPA followed by 2% CHX as an MMP inhibitor. (B) Etched with 37% OPA followed by 100% amla extract as an MMP inhibitor. (C) Etched with pure amla juice followed by 100% amla extract as an MMP inhibitor.OPA, orthophosphoric acid; CHX, chlorhexidine; MMP, matrix metalloproteinase.
|




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download