1. Rosenberg PA. Clinical strategies for managing endodontic pain. Endod Topics. 2002; 3:78–92.

2. Sathorn C, Parashos P, Messer H. The prevalence of postoperative pain and flare-up in single- and multiple-visit endodontic treatment: a systematic review. Int Endod J. 2008; 41:91–99. PMID:
17956561.

3. Ferraz CC, Gomes NV, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. Apical extrusion of debris and irrigants using two hand and three engine-driven instrumentation techniques. Int Endod J. 2001; 34:354–358. PMID:
11482718.

4. Bürklein S, Schäfer E. Apically extruded debris with reciprocating single-file and full-sequence rotary instrumentation systems. J Endod. 2012; 38:850–852. PMID:
22595125.

5. Tanalp J, Güngör T. Apical extrusion of debris: a literature review of an inherent occurrence during root canal treatment. Int Endod J. 2014; 47:211–221. PMID:
23711187.

6. Peuhkuri K, Nevala R, Vapaatalo H, Moilanen E, Korpela R. Ibuprofen augments gastrointestinal symptoms in lactose maldigesters during a lactose tolerance test. Aliment Pharmacol Ther. 1999; 13:1227–1233. PMID:
10468706.

7. Püspök A, Kiener HP, Oberhuber G. Clinical, endoscopic, and histologic spectrum of nonsteroidal anti-inflammatory drug-induced lesions in the colon. Dis Colon Rectum. 2000; 43:685–691. PMID:
10826432.

8. Etienney I, Beaugerie L, Viboud C, Flahault A. Non-steroidal anti-inflammatory drugs as a risk factor for acute diarrhoea: a case crossover study. Gut. 2003; 52:260–263. PMID:
12524410.

9. Khan AZ, George K, DeFriend DJ. Nonsteroidal anti-inflammatory drug-induced colonic stenosis: an unusual cause of a right-sided colonic mass: report of a case. Dis Colon Rectum. 2003; 46:403–405. PMID:
12626918.

10. Belitsky RB, Odam SJ, Hubley-Kozey C. Evaluation of the effectiveness of wet ice, dry ice, and cryogenic packs in reducing skin temperature. Phys Ther. 1987; 67:1080–1084. PMID:
3602101.

11. Modabber A, Rana M, Ghassemi A, Gerressen M, Gellrich NC, Hölzle F, Rana M. Three-dimensional evaluation of postoperative swelling in treatment of zygomatic bone fractures using two different cooling therapy methods: a randomized, observer-blind, prospective study. Trials. 2013; 14:238. PMID:
23895539.

12. Doğanay Yıldız E, Arslan H. Effect of low-level laser therapy on postoperative pain in molars with symptomatic apical periodontitis: a randomized placebo-controlled clinical trial. J Endod. 2018; 44:1610–1615. PMID:
30144985.

13. Coelho MS, Vilas-Boas L, Tawil PZ. The effects of photodynamic therapy on postoperative pain in teeth with necrotic pulps. Photodiagnosis Photodyn Ther. 2019; 27:396–401. PMID:
31301436.

14. Fayyad DM, Abdelsalam N, Hashem N. Cryotherapy: a new paradigm of treatment in endodontics. J Endod. 2020; 46:936–942. PMID:
32386857.

15. Swenson C, Swärd L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996; 6:193–200. PMID:
8896090.

16. Watkins AA, Johnson TV, Shrewsberry AB, Nourparvar P, Madni T, Watkins CJ, Feingold PL, Kooby DA, Maithel SK, Staley CA, Master VA. Ice packs reduce postoperative midline incision pain and narcotic use: a randomized controlled trial. J Am Coll Surg. 2014; 219:511–517. PMID:
25081937.

17. McDowell JH, McFarland EG, Nalli BJ. Use of cryotherapy for orthopaedic patients. Orthop Nurs. 1994; 13:21–30.

18. Kwekkeboom KL. Pain management strategies used by patients with breast and gynecologic cancer with postoperative pain. Cancer Nurs. 2001; 24:378–386. PMID:
11605708.

19. Koç M, Tez M, Yoldaş O, Dizen H, Göçmen E. Cooling for the reduction of postoperative pain: prospective randomized study. Hernia. 2006; 10:184–186. PMID:
16432641.

20. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004; 32:251–261. PMID:
14754753.

21. Forsgren H, Heimdahl A, Johansson B, Krekmanov L. Effect of application of cold dressings on the postoperative course in oral surgery. Int J Oral Surg. 1985; 14:223–228. PMID:
3926665.

22. Laureano Filho JR, de Oliveira e Silva ED, Batista CI, Gouveia FM. The influence of cryotherapy on reduction of swelling, pain and trismus after third-molar extraction: a preliminary study. J Am Dent Assoc. 2005; 136:774–778. PMID:
16022042.
23. Hubbard TJ, Denegar CR. Does cryotherapy improve outcomes with soft tissue injury? J Athl Train. 2004; 39:278–279. PMID:
15496998.
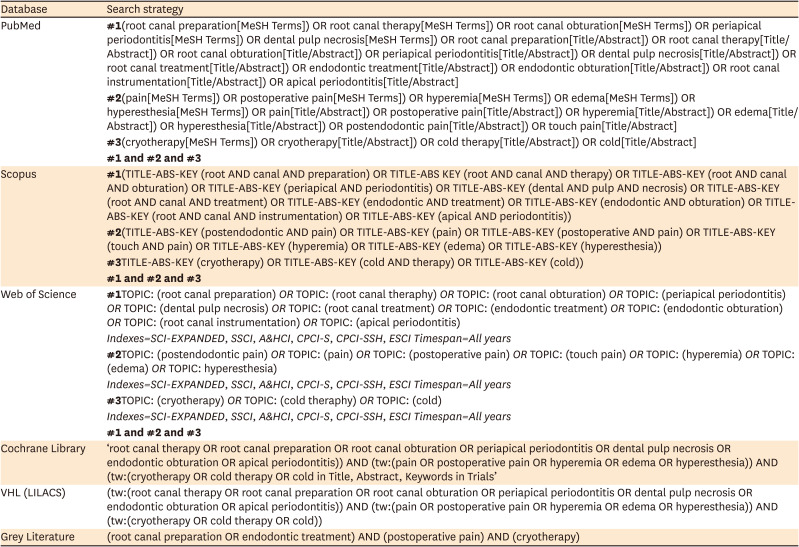
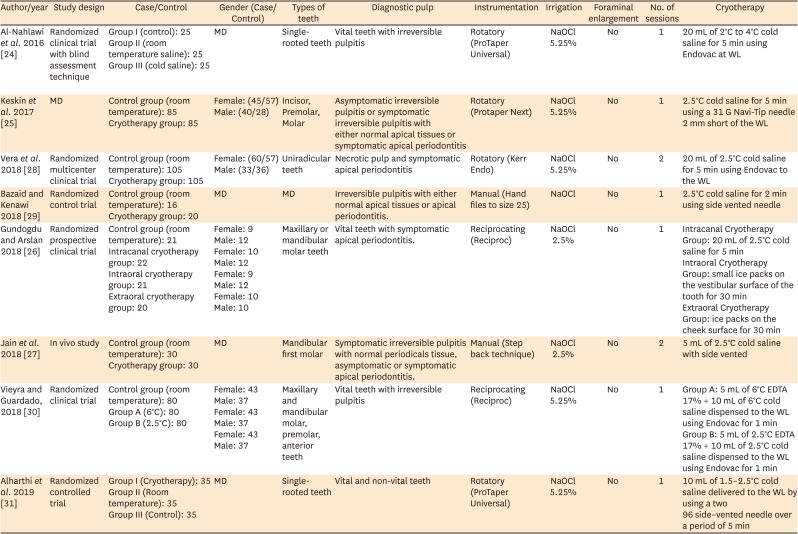
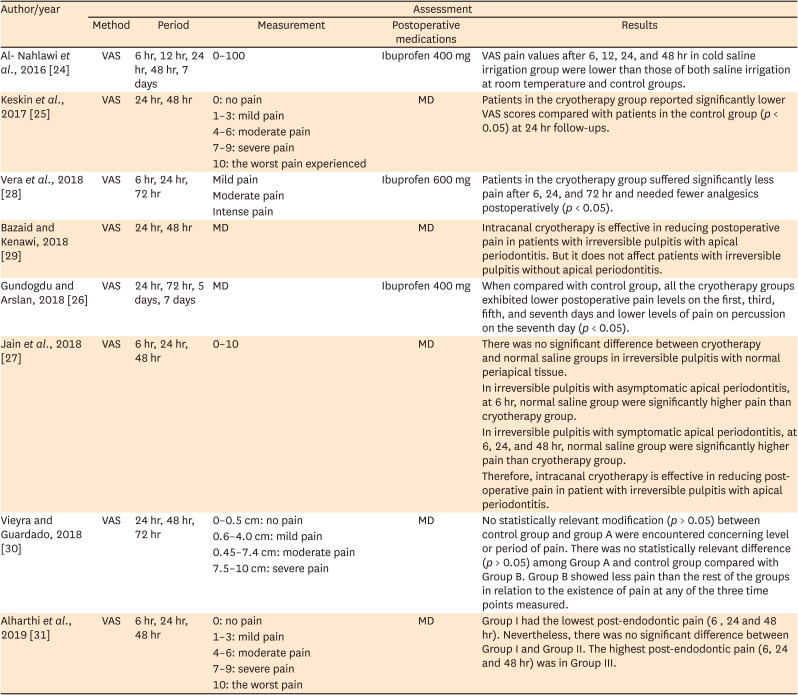
24. Al-Nahlawi T, Hatab TA, Alrazak MA, Al-Abdullah A. Effect of intracanal cryotherapy and negative irrigation technique on postendodontic pain. J Contemp Dent Pract. 2016; 17:990–996. PMID:
27965485.

25. Keskin C, Özdemir Ö, Uzun İ, Güler B. Effect of intracanal cryotherapy on pain after single-visit root canal treatment. Aust Endod J. 2017; 43:83–88. PMID:
27699913.

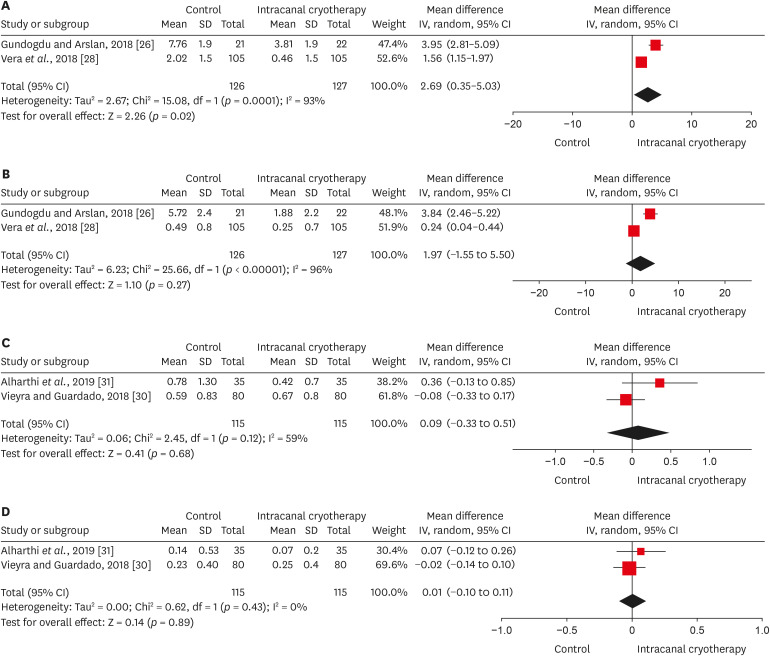
26. Gundogdu EC, Arslan H. Effects of various cryotherapy applications on postoperative pain in molar teeth with symptomatic apical periodontitis: a preliminary randomized prospective clinical trial. J Endod. 2018; 44:349–354. PMID:
29398090.

27. Jain A, Davis D, Bahuguna R, Agrawal A, Singh S, Ramachandran R, Varguese A. Role of cryotherapy in reducing postoperative pain in patients with irreversible pulpitis: an in-vivo study. Int J Dent Med Sci Res. 2018; 2:43–49.
28. Vera J, Ochoa J, Romero M, Vazquez-Carcaño M, Ramos-Gregorio CO, Aguilar RR, Cruz A, Sleiman P, Arias A. Intracanal cryotherapy reduces postoperative pain in teeth with symptomatic apical periodontitis: a randomized multicenter clinical trial. J Endod. 2018; 44:4–8. PMID:
29079057.

29. Bazaid DS, Kenawi LM. The effect of intracanal cryotherapy in reducing postoperative pain in patients with irreversible pulpitis: a randomized control trial. Int J Health Sci Res. 2018; 8:83–88.
30. Vieyra JP, Guardado JA. Reduction of post-endodontic pain after one-visit root canal treatment using three cryotherapy protocols with different temperature. Ann Materials Sci Eng. 2018; 3:1033.
31. Alharthi AA, Aljoudi MH, Almaliki MN, Almalki MA, Sunbul MA. Effect of intra-canal cryotherapy on post-endodontic pain in single-visit RCT: a randomized controlled trial. Saudi Dent J. 2019; 31:330–335. PMID:
31337936.

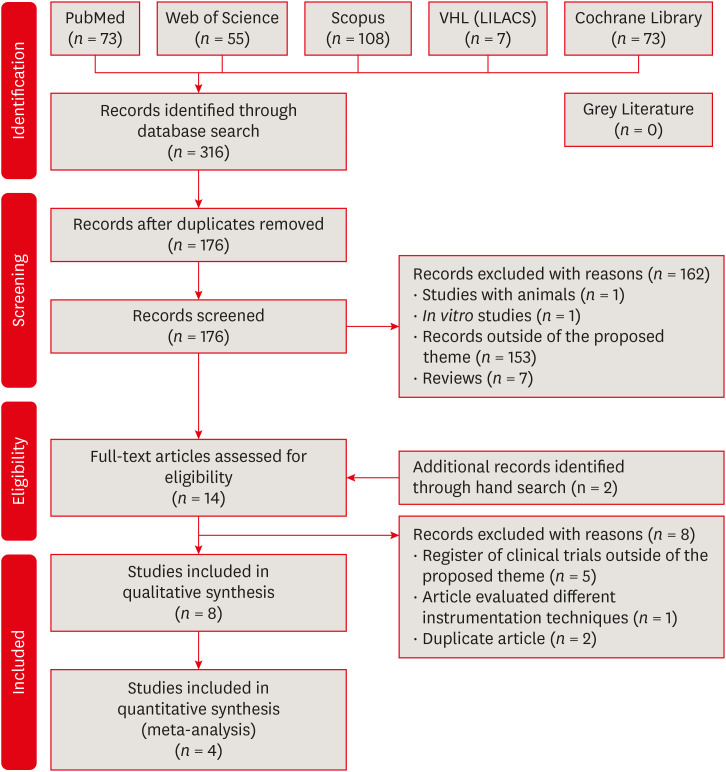
32. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269. PMID:
19622511.

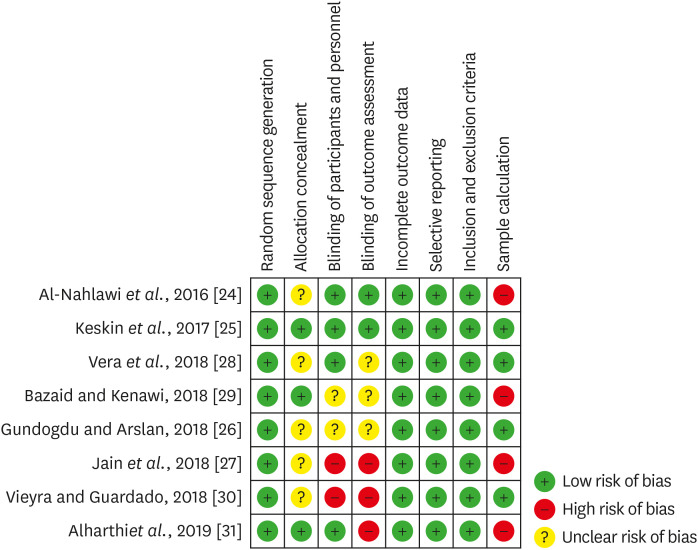
33. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Statistical Methods Group. Cochrane Bias Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID:
22008217.

34. Deeks JJ, Higgins JP. Chapter 10: Analysing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons;2019. p. 243–296.
35. Ammari MM, Soviero VM, da Silva Fidalgo TK, Lenzi M, Ferreira DM, Mattos CT, de Souza IP, Maia LC. Is non-cavitated proximal lesion sealing an effective method for caries control in primary and permanent teeth? A systematic review and meta-analysis. J Dent. 2014; 42:1217–1227. PMID:
25066832.

36. Borges Silva EA, Guimarães LS, Küchler EC, Antunes LA, Antunes LS. Evaluation of effect of foraminal enlargement of necrotic teeth on postoperative symptoms: a systematic review and meta-analysis. J Endod. 2017; 43:1969–1977. PMID:
29033088.

37. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010; 340:c869. PMID:
20332511.

38. Varela P, Souza E, de Deus G, Duran-Sindreu F, Mercadé M. Effectiveness of complementary irrigation routines in debriding pulp tissue from root canals instrumented with a single reciprocating file. Int Endod J. 2019; 52:475–483. PMID:
30317653.

39. Zehnder M. Root canal irrigants. J Endod. 2006; 32:389–398. PMID:
16631834.

40. Zehnder M, Kosicki D, Luder H, Sener B, Waltimo T. Tissue-dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:756–762. PMID:
12464903.

41. Gernhardt CR, Eppendorf K, Kozlowski A, Brandt M. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int Endod J. 2004; 37:272–280. PMID:
15056354.

42. de Sermeño RF, da Silva LA, Herrera H, Herrera H, Silva RA, Leonardo MR. Tissue damage after sodium hypochlorite extrusion during root canal treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:e46–e49.

43. Silva EJ, Menaged K, Ajuz N, Monteiro MR, Coutinho-Filho TS. Postoperative pain after foraminal enlargement in anterior teeth with necrosis and apical periodontitis: a prospective and randomized clinical trial. J Endod. 2013; 39:173–176. PMID:
23321226.

44. Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006; 15(Suppl 1):S17–S24. PMID:
16320034.

45. Nair PN. Apical periodontitis: a dynamic encounter between root canal infection and host response. Periodontol 2000. 1997; 13:121–148. PMID:
9567926.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download