1. Vernal R, Dezerega A, Dutzan N, Chaparro A, León R, Chandía S, Silva A, Gamonal J. RANKL in human periapical granuloma: possible involvement in periapical bone destruction. Oral Dis. 2006; 12:283–289. PMID:
16700737.

2. Ledesma-Montes C, Garcés-Ortíz M, Rosales-García G, Hernández-Guerrero JC. Importance of mast cells in human periapical inflammatory lesions. J Endod. 2004; 30:855–859. PMID:
15564863.

3. Marçal JR, Samuel RO, Fernandes D, de Araujo MS, Napimoga MH, Pereira SA, Clemente-Napimoga JT, Alves PM, Mattar R, Rodrigues V Jr, Rodrigues DB. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010; 36:995–999. PMID:
20478453.

4. Syed Ismail PM, Apoorva K, Manasa N, Rama Krishna R, Bhowmick S, Jain S. Clinical, radiographic, and histological findings of chronic inflammatory periapical lesions: a clinical study. J Family Med Prim Care. 2020; 9:235–238. PMID:
32110596.

5. Natanasabapathy V, Arul B, Mishra A, Varghese A, Padmanaban S, Elango S, Arockiam S. Ultrasound imaging for the differential diagnosis of periapical lesions of endodontic origin in comparison with histopathology: a systematic review and meta-analysis. Int Endod J. 2021; 54:693–711. PMID:
33368404.

6. Juerchott A, Pfefferle T, Flechtenmacher C, Mente J, Bendszus M, Heiland S, Hilgenfeld T. Differentiation of periapical granulomas and cysts by using dental MRI: a pilot study. Int J Oral Sci. 2018; 10:17. PMID:
29777107.

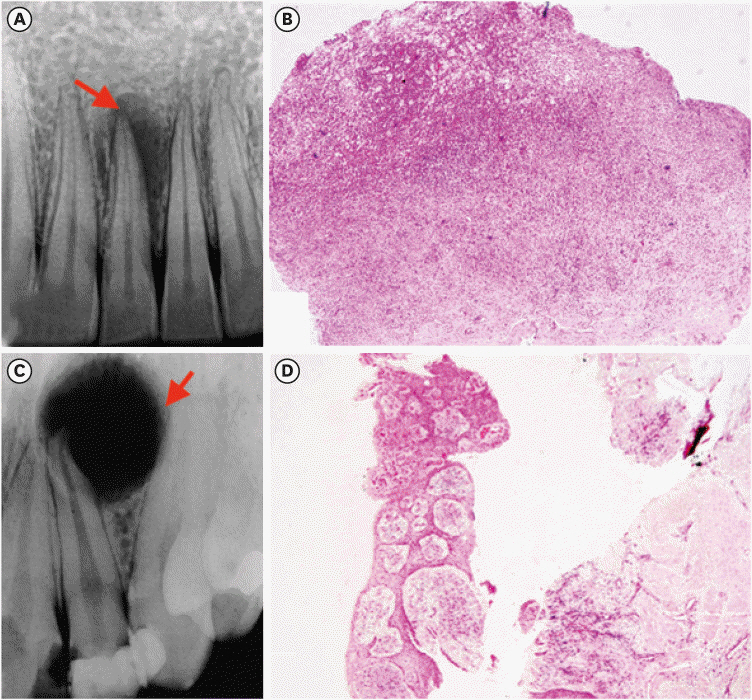
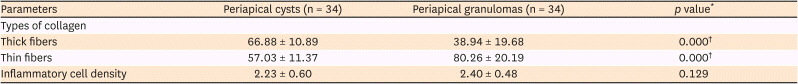
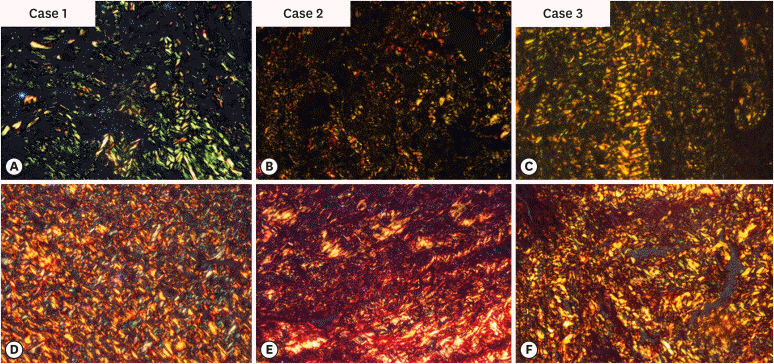
7. Ji HJ, Park SH, Cho KM, Lee SK, Kim JW. Differential diagnosis of periapical cyst using collagen birefringence pattern of the cyst wall. Restor Dent Endod. 2017; 42:111–117. PMID:
28503476.

8. Jahagirdar PB, Kale AD, Hallikerimath S. Stromal characterization and comparison of odontogenic cysts and odontogenic tumors using picrosirius red stain and polarizing microscopy: A retrospective and histochemical study. Indian J Cancer. 2015; 52:408–412. PMID:
26905155.

9. El Safoury OS, Fawzy MM, El Maadawa ZM, Mohamed DH. Quantitation of mast cells and collagen fibers in skin tags. Indian J Dermatol. 2009; 54:319–322. PMID:
20101330.

10. Vij R, Vij H, Rao NN. Evaluation of collagen in connective tissue walls of odontogenic cysts--a histochemical study. J Oral Pathol Med. 2011; 40:257–262. PMID:
20969631.

11. França GM, Carmo AF, Costa Neto H, Andrade AL, Lima KC, Galvão HC. Macrophages subpopulations in chronic periapical lesions according to clinical and morphological aspects. Braz Oral Res. 2019; 33:e047. PMID:
31141038.

12. Yong LC. The mast cell: origin, morphology, distribution, and function. Exp Toxicol Pathol. 1997; 49:409–424. PMID:
9495641.

13. Costa Neto H, de Andrade AL, Gordón-Núñez MA, Freitas RA, Galvão HC. Immunoexpression of tryptase-positive mast cells in periapical granulomas and radicular cysts. Int Endod J. 2015; 48:729–735. PMID:
25100244.

14. Andrade AL, Santos EM, Carmo AF, Freitas RA, Galvão HC. Analysis of tryptase-positive mast cells and immunoexpression of MMP-9 and MMP-13 in periapical lesions. Int Endod J. 2017; 50:446–454. PMID:
27003572.

15. Floratos S, Kim S. Modern Endodontic Microsurgery Concepts: A Clinical Update. Dent Clin North Am. 2017; 61:81–91. PMID:
27912820.
16. Ankle MR, Joshi PS. A study to evaluate the efficacy of xylene-free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: an experimental study. J Oral Maxillofac Pathol. 2011; 15:161–167. PMID:
22529574.

17. Hirshberg A, Lib M, Kozlovsky A, Kaplan I. The influence of inflammation on the polarization colors of collagen fibers in the wall of odontogenic keratocyst. Oral Oncol. 2007; 43:278–282. PMID:
16919995.

18. Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. 1973; 150:174–187. PMID:
4129194.

19. Hirshberg A, Sherman S, Buchner A, Dayan D. Collagen fibres in the wall of odontogenic keratocysts: a study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1999; 28:410–412. PMID:
10535364.

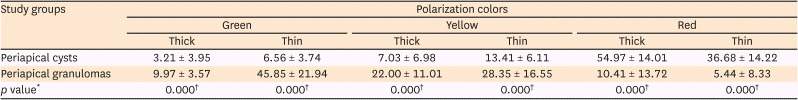
20. Shetty A, Tamgadge A, Bhalerao S, et al. Study of polarization colors in the connective tissue wall of odontogenic cysts using picrosirius red stain. J Orofac Sci. 2015; 7:119–124.

21. Singh HP, Shetty DC, Wadhwan V, Aggarwal P. A quantitative and qualitative comparative analysis of collagen fibers to determine the role of connective tissue stroma on biological behavior of odontogenic cysts: a histochemical study. Natl J Maxillofac Surg. 2012; 3:15–20. PMID:
23251052.

22. Mahajan AM, Mahajan MC, Ganvir SM, Hazarey VK. The role of stroma in the expansion of odontogenic cysts and adenomatoid odontogenic tumor: a polarized microscopy study. J Nat Sci Biol Med. 2013; 4:316–320. PMID:
24082724.

23. Aggarwal P, Saxena S. Stromal differences in odontogenic cysts of a common histopathogenesis but with different biological behavior: a study with picrosirius red and polarizing microscopy. Indian J Cancer. 2011; 48:211–215. PMID:
21768668.

24. Sreela KK, Cherian LM, Beena VT, Heera R. Comparison of picrosirius red staining of collagen fibers of Keratocystic odontogenic tumor with odontogenic cysts. IOSR J Dent Med Sci. 2017; 16:45–54.
25. Szendröi M, Vajta G, Kovács L, Schaff Z, Lapis K. Polarization colours of collagen fibres: a sign of collagen production activity in fibrotic processes. Acta Morphol Hung. 1984; 32:47–55. PMID:
6431760.
26. Bergamini ML, Mardegan AP, DE Rosa CS, Palmieri M, Sarmento DJ, Hiraki KR, Costa AL, HassÉus B, Jonasson P, Braz-Silva PH. Presence of langerhans cells, regulatory T cells (Treg) and mast cells in asymptomatic apical periodontitis. Braz Oral Res. 2020; 34:e108. PMID:
32876121.
27. de Oliveira Rodini C, Batista AC, Lara VS. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: possible role of mast cells in the course of human periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:59–63. PMID:
14716257.

28. Drazić R, Sopta J, Minić AJ. Mast cells in periapical lesions: potential role in their pathogenesis. J Oral Pathol Med. 2010; 39:257–262. PMID:
20359310.

29. Fonseca-Silva T, Santos CC, Alves LR, Dias LC, Brito M Jr, De Paula AM, Guimarães AL. Detection and quantification of mast cell, vascular endothelial growth factor, and microvessel density in human inflammatory periapical cysts and granulomas. Int Endod J. 2012; 45:859–864. PMID:
22486765.

30. Mahita VN, Manjunatha BS, Shah R, Astekar M, Purohit S, Kovvuru S. Quantification and localization of mast cells in periapical lesions. Ann Med Health Sci Res. 2015; 5:115–118. PMID:
25861530.

32. Thakur SK, Thakur R, Sankhyan A, Patyal A. A conservative approach to treat large periapical lesions: a report of two cases. Indian J Dent Sci. 2019; 11:225–228.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download