Abstract
Epilepsy surgery is a well-established treatment for drug-resistant epilepsy, with awake craniotomy being used in certain cases to remove epileptogenic foci while preserving crucial brain functions. We are presenting the first reported case from Pakistan of a 19-year-old woman who underwent awake epilepsy surgery to treat cortical dysplasia. She had a history of generalized tonic-clonic seizures since her childhood and was referred to our clinic due to an increase in seizure frequency. EEG and MRI identified the epileptogenic focus in the right parieto-temporal region. The patient underwent a neuro-navigation guided awake craniotomy and an excision of the epileptogenic focus in the right parieto-temporal region. The procedure was carried out using a scalp block and dexmedetomidine for conscious sedation, enabling the patient to remain awake throughout the surgery. Intraoperative mapping and electrocorticography were used for complex multidisciplinary care. Post-resection corticography showed no spikes along the resected margins. The patient was discharged without any complications and remained free of symptoms a year after the surgery. Awake epilepsy surgery is a viable option for removing epileptogenic foci while preserving vital cognitive functions. However, it is seldom used in low- and middle-income countries such as Pakistan. The successful outcome of this case underscores the need for greater awareness and availability of epilepsy surgery in resource-limited settings. Cost-effective measures, such as using small subdural strips for intraoperative localization, can be implemented.
Awake craniotomy (AC) is increasingly being recognized as the standard of care for the resection of lesions in eloquent areas [12]. Although intraoperative monitoring of neurological function is the most significant advantage, additional benefits include the avoidance of general anesthesia, a shorter recovery period, and potentially lower costs [12]. These factors have contributed to expanding the limits of resection, with an enhanced comprehension of the patient-specific functional organization of the cortex. Epilepsy surgery includes a wide range of treatment alternatives, with either curative or palliative objectives. Lesional epilepsy, when unresponsive to medical treatment, necessitates surgical resection of the epileptogenic zone. Accurate identification of the epileptogenic zone, using a combination of electrophysiological and radiological findings, is essential. Intraoperative electrocorticography (ECoG) assists in delineating the target zone. AC facilitates brain mapping and monitoring in cases where the lesion is situated in an eloquent area, allowing for maximum safe resection. Lesions near the eloquent cortex require precise dissection between functional normal and epileptogenic tissue. Epileptogenic regions often remain undetected on imaging and exhibit subtle histological changes [1]. However, when the lesion involves an eloquent area, its identification becomes a critical task for the surgeon [2].
While the use of AC for epilepsy surgery has been a standard practice in the developed world for a significant amount of time, it is still a relatively new approach in low- and middle-income countries. Pakistan, being the 5th most populous country in the world, has a high incidence of epilepsy. However, there have been no reports of AC being used for epilepsy surgery in this region to date. We are reporting the first successful case of an AC for lesionectomy in a patient with refractory epilepsy, with a follow-up period of 1 year.
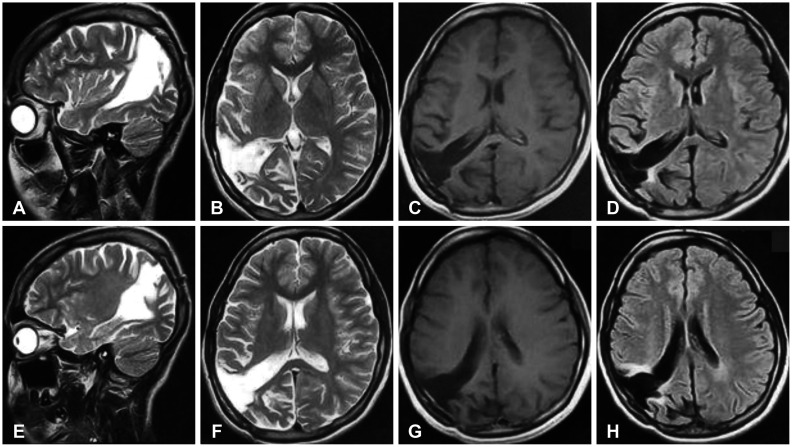
The patient is a 19-year-old, right-handed woman who was admitted by choice due to a history of generalized tonic-clonic seizures dating back to when she was 5 years old. Her seizures were initially controlled by medication, but over time, they increased in frequency and became resistant to multiple antiepileptics. Upon admission, she was experiencing 3 to 6 episodes of generalized tonic-clonic seizures daily. Her neurological assessment was otherwise normal. An epileptologist initially evaluated her and recommended a 48-hour video electroencephalography (EEG). Six separate clinical seizures were observed on the video EEG, with slow theta activity in the right parietotemporal region seen preceding each event. Faster beta activity was observed on the right side, spreading to the left side. The majority of the patient’s seizures originated from the right parietotemporal (F4, P4, T2, T4, T6) region, with occasional left temporoparietal irritability. MRI revealed hyperintense signals on FLAIR sequences in the right posterior parietal and temporal lobes, without post-contrast enhancement (Fig. 1). On diffusion tensor imaging, the lesion was seen abutting the adjacent superior longitudinal fasciculus in the posterior parietal lobe, without infiltration.
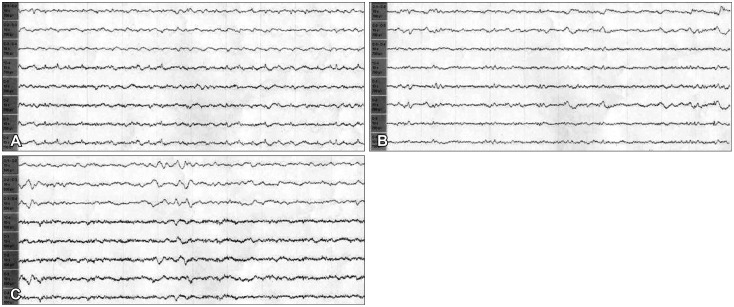
She underwent a neuro-navigation guided AC and removal of the epileptogenic focus. A multidisciplinary team consisting of neurosurgeons, neuro-anesthesiologists, an epileptologist, and neurophysiologists was involved in complex care, carrying out intraoperative mapping and ECoG. The anesthesiologist planned for the patient to remain conscious throughout the procedure via a scalp block and the use of dexmedetomidine for conscious sedation. In addition to routine monitoring specified by the American Society of Anesthesiologists (ASA), a bispectral index monitor (BIS) was also employed. A scalp block was performed using ropivacaine 0.5% and an anatomical landmark technique. Dexmedetomidine infusion was initiated for conscious sedation at a rate of 0.2 to 0.5 µg/kg/h, as guided by the BIS, which was maintained between 70 and 85. The patient was administered 1 g of levetiracetam and 200 mg of lacosamide. Following the craniotomy and durotomy, electrical corticography was performed using the Ojemann grid. All epileptogenic foci were marked as depicted in Figs. 2, 3, 4. The gray matter was indistinguishable from abnormal brain tissue. The resection margin extended to the parieto-occipital sulcus and superior temporal gyrus. Even though the resected cortex was hard and firm, the frozen section did not align with a glioma diagnosis. Post-resection, corticography revealed no spikes along the resected margins, as shown in Fig. 5. Throughout the procedure, the patient’s speech and motor functions were continuously monitored; there were no intraoperative deficits noted.
The patient was transferred to the recovery unit with stable hemodynamics, and her pain was consistently managed throughout the perioperative period. Antiepileptic medications were resumed after the operation, and she was discharged to her home on the second day following the procedure without any complications during her hospital stay. The final histopathology report indicated focal cortical dysplasia. The patient has remained free of seizures after a 1-year follow-up in the clinic. Fig. 6 displays the post-resection MRI. She continues to take the same dosage of antiepileptic drugs as she was prescribed at the time of her surgery.
Focal cortical dysplasias (FCDs) are malformations in cortical development with a high potential for causing epilepsy. FCDs are commonly associated with drug-resistant epilepsy and are the most common cause of intractable epilepsy in children requiring epilepsy surgery [3]. The success rate of epilepsy surgery varies between 50% and 75%, largely depending on the accuracy with which neurosurgeons can locate and remove the identified focus. Complete resection is generally accepted as the most important factor in prognostication [4]. However, the lack of a clear boundary between the epileptogenic zone and the eloquent cortex often results in incomplete resection [5].
Awake craniotomy has traditionally been the best approach in these scenarios. Patients with epileptogenic zones located in language areas, previously only considered for palliative measures, can now be entirely disease-free [2]. A recent series of studies concluded that awake epilepsy surgery in patients with FCDs affecting the eloquent cortex resulted in satisfactory seizure control in comparison to general anesthesia plus intra-operative neuro-monitoring with FCDs affecting motor cortex, and with FCDs outside the eloquent areas operated on under general anesthesia only [6]. Awake craniotomy also provides additional information about the lesion in the eloquent areas during the surgery, which can alter the perioperative plan. Awake surgery allows for language, primary motor, and sensory mapping, which assists in determining resectability. Epilepsy surgery within eloquent regions was previously considered feasible but difficult to achieve complete seizure control and carried an increased risk of complications. Awake epilepsy surgery, however, has been reported to control seizures satisfactorily in many cohorts, with low rates of permanent deficits, even in cases of non-lesional epilepsy.
Language mapping is difficult to predict in patients with epilepsy as reorganization of these areas is often observed, both interhemispheric and intrahemispheric. FCDs are believed to develop during the prenatal period, a time of neuroplasticity and remodeling. Identifying reorganized functional regions can allow for maximum resection of FCDs with minimal intraoperative complications.
In the Pakistani population, the incidence of epilepsy is approximately 9.9 per 1,000 individuals, predominantly affecting the younger population [7]. Given the severity and impact of the disease, surgical options for drug-resistant epilepsy should be considered in selected cases. Our patient, for instance, was unable to continue her education due to intractable epilepsy, significantly diminishing her quality of life.
Neuro-anesthesiologists play an essential role in safe monitoring through an awake-throughout approach. This technique is well-tolerated, particularly with careful patient selection and psychological preparation [8]. Dexmedetomidine is an ideal choice for conscious sedation due to its rapid awakening properties and its ability to allow successful mapping of motor functions and speech, in comparison to propofol [9]. Dexmedetomidine does not affect epileptic activity, making it suitable for use during intraoperative corticography in epilepsy surgery [10].
A recent meta-analysis of randomized controlled trials comparing dexmedetomidine with propofol for systemic sedation in AC observed better surgeon satisfaction with dexmedetomidine [11]. This can be attributed to dexmedetomidine allowing patients to easily transition from sedated to awake states and perform neurophysiological tasks smoothly. This method is beneficial in allowing high-quality intraoperative brain mapping with lower risk of respiratory depression compared to a classical propofol-remifentanil-based sedation in supratentorial surgery.
There is limited literature reporting awake epilepsy surgery from low- and middle-income countries. This may be due to limited uptake of epilepsy surgery by medical practitioners and patients, as well as limited resources to develop long-term programs. International twinning programs have proven successful in developing comprehensive epilepsy surgery centers in developing nations [12]. The benefits of AC for epilepsy surgery could help patients achieve good long-term seizure control; our case report demonstrates a practical approach to implementing these techniques. Moreover, the use of a small strip to determine lesion margins, rather than a larger, more expensive grid, proved effective for achieving postoperative seizure control. This requires coordination between the surgeon and epileptologist to help interpret intraoperative corticography as it is being performed in real time. Cost-effectiveness is a significant barrier to epilepsy surgery, which is particularly exacerbated in resource-limited centers [13].
In conclusion, surgery for epileptogenic foci is a feasible and safe alternative when the disease becomes resistant to traditional medical treatment. A maximum safe removal is crucial for the long-term reduction in seizure frequency and dependency on antiepileptic drugs. Awake epilepsy surgery allows for neuro-monitoring with maximum safe removal. Although documented in the literature, AC for epilepsy surgery is still underutilized in our region. There is a need for further expansion of awake epilepsy surgery within our region, as well as increased awareness among patients and non-surgeon providers about the effectiveness and safety of this procedure.
Notes
Ethics Statement: The IRB exempted patient’s informed consent as long as patient’s identity is not disclosed or compromised.
Author Contributions:
Data curation: all authors.
Project administration: Syed Ather Enam, Saqib Kamran Bakhshi, Faraz Shafiq.
Supervision: Syed Ather Enam, Saqib Kamran Bakhshi, Faraz Shafiq.
Writing—original draft: Mohammad Hamza Bajwa, Syeda Amrah Hashmi, Abdullah Nisar, Muhammad Waqas Baqai, Saqib Kamran Bakhshi, Muskaan Abdul Qadir.
Writing—review & editing: Syed Ather Enam, Saqib Kamran Bakhshi, Faraz Shafiq.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
1. Maesawa S, Nakatsubo D, Fujii M, Iijima K, Kato S, Ishizaki T, et al. Application of awake surgery for epilepsy in clinical practice. Neurol Med Chir (Tokyo). 2018; 58:442–452. PMID: 30249918.

2. Korkar GH, Isnard J, Montavont A, Catenoix H, Rheims S, Guénot M. Awake craniotomy for epilepsy surgery on eloquent speech areas: a single-centre experience. Epileptic Disord. 2021; 23:347–356. PMID: 33926856.

3. Choi SA, Kim KJ. The surgical and cognitive outcomes of focal cortical dysplasia. J Korean Neurosurg Soc. 2019; 62:321–327. PMID: 31085958.

4. Hauptman JS, Mathern GW. Surgical treatment of epilepsy associated with cortical dysplasia: 2012 update. Epilepsia. 2012; 53(Suppl 4):98–104.

5. Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Long-term outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg. 2004; 101:55–65. PMID: 15255252.

6. Minkin K, Gabrovski K, Karazapryanov P, Milenova Y, Sirakov S, Karakostov V, et al. Awake epilepsy surgery in patients with focal cortical dysplasia. World Neurosurg. 2021; 151:e257–e264. PMID: 33872840.

7. Khatri IA, Iannaccone ST, Ilyas MS, Abdullah M, Saleem S. Epidemiology of epilepsy in Pakistan: review of literature. J Pak Med Assoc. 2003; 53:594–597. PMID: 14765939.
8. Shafiq F, Parkash J, Enam A, Khan MF, Baig T. An awake throughout approach for awake craniotomy: a perspective from a resource-limited country. World Neurosurg. 2019; 126:e1489–e1493. PMID: 30905650.

9. Shen SL, Zheng JY, Zhang J, Wang WY, Jin T, Zhu J, et al. Comparison of dexmedetomidine and propofol for conscious sedation in awake craniotomy: a prospective, double-blind, randomized, and controlled clinical trial. Ann Pharmacother. 2013; 47:1391–1399. PMID: 24259599.

10. Souter MJ, Rozet I, Ojemann JG, Souter KJ, Holmes MD, Lee L, et al. Dexmedetomidine sedation during awake craniotomy for seizure resection: effects on electrocorticography. J Neurosurg Anesthesiol. 2007; 19:38–44. PMID: 17198099.

11. Viderman D, Nabidollayeva F, Bilotta F, Abdildin YG. Comparison of dexmedetomidine and propofol for sedation in awake craniotomy: a meta-analysis. Clin Neurol Neurosurg. 2023; 226:107623. PMID: 36791589.

12. Tahir MZ, Sobani ZA, Quadri SA, Ahmed SN, Sheerani M, Siddiqui F, et al. Establishment of a comprehensive epilepsy center in Pakistan: initial experiences, results, and reflections. Epilepsy Res Treat. 2012; 2012:547382. PMID: 22957232.

13. Thuy Le MA, Fong SL, Lim KS, Gunadharma S, Sejahtera DP, Visudtibhan A, et al. Underutilization of epilepsy surgery in ASEAN countries. Seizure. 2019; 69:51–56. PMID: 30974407.

Fig. 1
Preoperative MRI of the lesion in sagittal (A, B, G, H), axial (C, D, I, J), coronal views (E, F, K, L), with T2-FLAIR and corresponding T1-post contrast sequences. The lesion shows few hyperintense regions on FLAIR imaging with no contrast enhancement. FLAIR, fluid-attenuated inversion recovery.
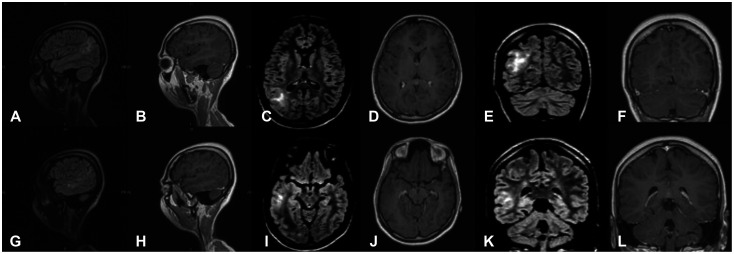
Fig. 2
Intraoperative delineation of lesion margins as determined on electrical corticography: medial margin (A), posterior margin (B), anterior margin (C), and lateral margin (D).
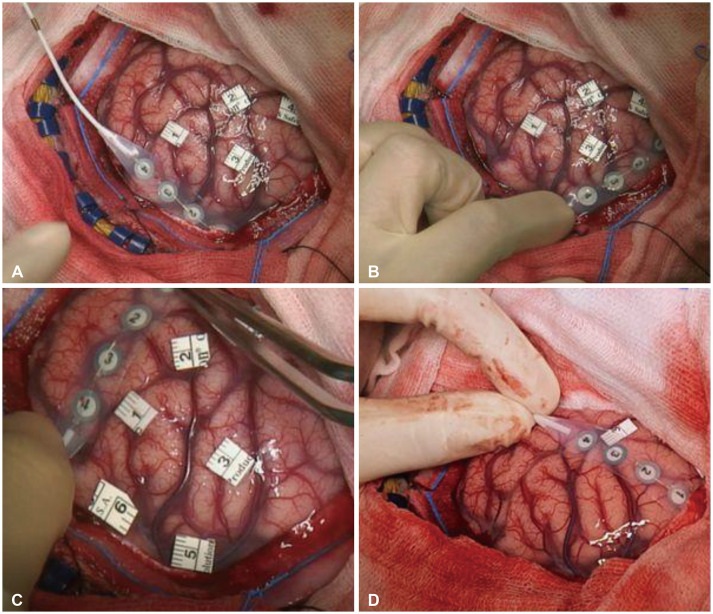
Fig. 3
Immediate pre-resection electroencephalography (EEG). A: Anterior margin of the epileptogenic zone EEG. B: Posterior margin of the epileptogenic zone EEG. These depict fast and spike wave patterns.
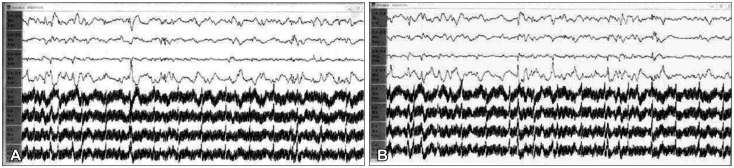
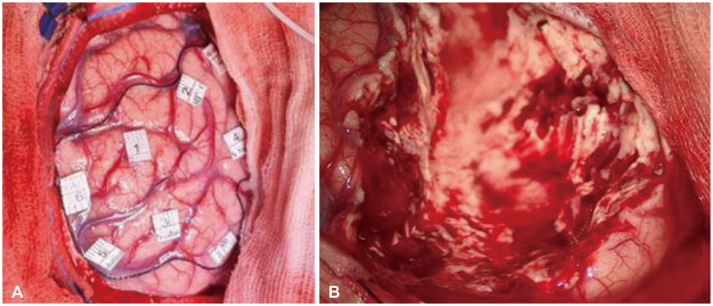
Fig. 4
Intraoperative images. A: Depicts margins of the epileptogenic zone identified by intraoperative electrocorticography, as marked by a black thread. B: Depicts immediate post-resection cavity.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download