Abstract
Background and Objectives
Head and neck cancer can occur as a second primary tumor in patients with a history of hematological malignancy, and the pathological features of those are more aggressive than de novo primary head and neck cancer. However, research related to the prognostic value of hematological markers in the head and neck cancer patients with a history of hematological malignancy is scarce, so we performed this study.
Subjects and Method
We reviewed a total of 89 head and neck cancer patients with hematological malignancy diagnosed at a single tertiary hospital between 1997 and 2021, and enrolled 29. The analyzed hematological parameters included pre- and post-treatment hemoglobin, hematocrit, lactate dehydrogenase, absolute neturophil count (ANC), absolute platelet count, absolute leukocyte count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio.
Results
Hematological malignancy was diagnosed almost 62.14±96.10 months before head and neck cancer. The most common hematological malignancy was acute myeloid leukemia. The overall survival (OS) rate was lower in patients with second primary tumor than in those with de novo primary head and neck cancer. The cox-proportional hazard ratio analysis showed that the post-treatment absolute neutrophil count was significantly correlated with disease-free survival but not with OS.
Hematological malignancies accounted for 10% of all cancers in the United States in 2019 [1]. In South Korea, non-Hodgkin’s lymphoma is the most common hematological malignancy, followed by leukemia and multiple myeloma [2]. A recent study reported a significant increase in all hematologic malignancies [3]. Furthermore, hematological malignancies increase the risk of a second primary tumor [4].
The common solid organs where second primary head and neck cancer with a history of hematological malignancy frequently occur is known to be the prostate, lung/bronchus, breast, and skin [5]. Particularly, it has been reported that in the case of Hodgkin lymphoma as primary hematological malignancy, lung cancer occurs frequently in male, while oral cavity & pharynx cancer occur frequently in female [6]. Moreover, the pathological features of second primary tumor with a history of hematological malignancy was known to be aggressive showing a significantly lower overall survival (OS) rate compared to primary tumor alone [7].
The prognostic factors for second primary tumor with a history of hematological malignancy include age, chemotherapy, bone marrow transplantation (BMT), and novel cytogenetic and molecular biology parameters [8]. However, studies on the prevalence and prognosis of head and neck cancer in patients with a history of hematological malignancy are yet insufficient. Furthermore, the research for serologic markers as a prognostic factor is scarce in second primary tumor with a history of hematological malignancy. So, we evaluated the prognostic value of hematological parameters for head and neck cancer patients with a history of hematological malignancy.
In this retrospective study, we reviewed the medical records of head and neck cancer patients who had a history of hematological malignancy and were diagnosed at Seoul St. Mary’s hospital between 1997 and 2021. We excluded patients with malignancies other than head and neck cancer and hematological malignancy, missing data for important serological parameters (such as complete blood count and blood chemistry), loss to follow-up before study completion, or death not related to the cancer of interest. We also excluded patients with lymphoma, to rule out any effect of lymphoproliferative disease when evaluating the prognostic value of hematological parameters for hematological malignancy. Among the total of 89 patients, 29 were included in the study. We also compared the survival status of 3192 head and neck cancer patients without a history of hematological malignancy diagnosed for the same period of this study to that of those with a history of hematological malignancy. Hematological malignancies, particularly acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML), chronic lymphoid leukemia (CLL), myelodysplastic syndrome (MDS), and head and neck cancer (including that affecting the cavity, oropharynx, hypopharynx, larynx, and other primary sites, such as salivary glands or nasal cavity), were classified using the 10th revision of the International Classification of Diseases and staged according to the 8th edition of the AJCC cancer staging system. Pre- and post-treatment blood samples were obtained at the first and final visits to our clinic during the study period, respectively.
We analyzed the prognostic value of pre- and post-treatment hemoglobin (Hb), hematocrit (Hct), and lactate dehydrogenase (LDH), and the absolute neutrophil count (ANC), absolute platelet count, absolute leukocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR). Previous studies have shown that a low Hb level and high LDH level correlate with the tumor burden in advanced hematological diseases [9]. Furthermore, LDH is a prognostic factor for non-Hodgkin lymphoma, and is included in the International Prognostic Index. The prognostic values of inflammation-related serological markers, such as the ANC, ALC, absolute platelet count, PLR, and NLR, were also analyzed. Several previous studies have shown that inflammation plays an important role in tumor development and progression [10]. Recently, Arend, et al. [11] reported that pre-treatment ANC negatively correlates with the risks of recurrence and mortality in cancers. Increased NLR can be used as an inflammatory marker to distinguish between low- and highgrade malignant head and neck cancer [12]. A recent study by Koyama, et al. [13] showed that ALC and PLR were significantly related to the OS and treatment efficacy in breast cancer. In the present study, we selected candidate peripheral blood hematological markers based on the previous studies.
Statistical analysis was performed using R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria). The log-rank test and Kaplan-Meier curves were used to compare OS and disease-free survival (DFS) between 29 and 3,192 head and neck cancer patients with and without a history of hematological malignancy, respectively. The cut-off values for the hematological parameters were calculated based on the maximum Youden index (sensitivity+specificity-1), obtained from receiver operating characteristic (ROC) curve analysis. Cox proportional hazard regression analysis was used to calculate the relative risk.
The Institutional Review Board (IRB) of our hospital approved this study (KC22RASI0535). The study was conducted in accordance with the relevant laws and regulations, good clinical practices, and ethical principles, as described in the Declaration of Helsinki.
Table 1 presents the characteristics of 29 head and neck cancer patients with a history of hematological malignancy. The mean ages at diagnosis of the hematological malignancy and head and neck cancer were 39.79±21.04 and 45.97±17.58 years, respectively. Hematological malignancy was diagnosed almost 62.14±96.10 months before head and neck cancer (p=0.003). Of the 29 patients, 21 (72.4%) were males, 12 (41.4%) were smokers, and 14 (48.3%) consumed alcohol. AML (n=11, 37.9%) was the most common hematological malignancy, followed by MDS (n=7, 24.1%), ALL (n=5, 17.2%), CML (n=5, 17.2%), and CLL (n=1, 3.4%). BMT was performed in 16 patients (55.2%), among whom 13 (44.8%) underwent HLA type matching. At the final follow-up, the mean survival period of the 29 patients was 3.93±4.45 years.
We compared prognosis and OS between head and neck cancer patients with and without a previous hematological malignancy (Fig. 1), and found a statistically significant difference in OS between the two groups (17.24% vs. 22.01%, respectively; p=0.044).
Table 2 presents the mean and standard deviation of the serological parameters for the hematological malignancy subgroups, and for hematological disease overall. The pre- and post-treatment Hb levels in the overall hematological malignancy group were 11.6±2.8 and 11.4±2.5 g/dL, respectively. ALL patients had the most severe pre-treatment anemia (10.8±3.5 g/dL). CML patients had anemia at the time of post-treatment (11.3±2.6 g/dL). Similar to Hb level, pre- and post-treatment Hct levels were lower in ALL and CML patients than in the other groups. However, there was no significant difference between the groups. The pre- and post-treatment LDH levels in the overall hematological malignancy group were 1180±3007 and 372±279 U/L, respectively, and the ANCs were 4113±6746×109/L and 4958±4704×109/L, respectively. There was also no significant difference in any other serological markers between the groups.
The ROC curve displays the sensitivity and 1-specificity; the area under the curve (AUC) is a useful indicator of accuracy [14]. Diagnostic tests with higher AUC values are more accurate, and the tests are considered reliable when the AUC value is >0.5 [15]. Among the serological parameters evaluated in the present study, pre-treatment Hb and Hct, post-treatment LDH, ANC, NLR, and pre- and post-treatment PLC were found to be reliable predictors of OS, with AUC values >0.5. The Youden index is a commonly used metric of the overall diagnostic effectiveness, and includes the sensitivity and specificity. The optimal cut-off value for a diagnostic test is determined by assigning equal weight to the sensitivity and specificity (i.e., maximizing the sensitivity+specificity value) [16]. Based on the maximum Youden index, the optimal cut-off values for each variable in this study were as follows: Hb, 13.8 g/dL; Hct, 40.4%; LDH, 259 U/L; ANC, 6365×109/L; NLR, 4.5; and pre-and post-treatment PLR, 101 and 92, respectively.
We evaluated the relationships of the aforementioned serological markers with OS and DFS in head and neck cancer patients with and without a history of hematological malignancy. There was no statistically significant association of the serological markers with OS (Table 3). Because the serological markers are often abnormal in patients with hematological malignancy, OS depended on hematological malignancy, rather than head and neck cancer. We also analyzed DFS to evaluate the effect of head and neck cancer. Only the post-treatment ANC was significantly correlated with the DFS (cut-off value=6365×109/L, hazard ratio [HR]=4.407, 95% confidence interval [CI]=1.036-18.508, p=0.048) (Table 4).
An increasing number of studies are being performed on the prevalence and pathogenesis of second primary tumors after hematological malignancy. Most studies found that the incidence of second primary tumor increased after hematological malignancy [4]. By contrast, some studies reported that the incidence of solid tumors, as a second primary malignancy after multiple myeloma, decreased [17]. Several studies have been performed on the pathogenesis of second primary tumor after hematological malignancy. One study reported that chemotherapy for hematological malignancy increased the risk of a second primary tumor by creating a selective pressure accelerating the clonal evolution of specific tissues [18]. Second primary malignancy may develop through various mechanisms, including an abnormal immune-mediated signaling cascade, genetic mutations, and T cell dysfunction [7]. Although numerous studies have evaluated the epidemiology and pathogenesis of second primary malignancy after hematological malignancy, studies on the prognosis are scarce. Therefore, we evaluated the prognostic factors of head and neck cancer patients with a history of hematological malignancy.
Several hematological parameters are useful prognostic markers in patients with a hematological malignancy. Chen, et al. [19] reported that the pre-treatment Hb level and monocyte and platelet count were independent prognostic factors, as tumor-related monocytosis, thrombocytosis, and anemia were associated with poor OS in head and neck squamous cell carcinoma patients. In our study, the pre-treatment Hct level and post-treatment LDH level, ANC, and NLR were negatively correlated with OS, although the results did not reach statistical significance (Table 3). These abnormal parameters are explained by bone marrow suppression due to the hematological malignancy, and may reflect an immunocompromised state; this would explain their negative association with OS. We evaluated the associations of hematological markers with DFS to exclude the effects of bone marrow suppression due to hematological malignancy in head and neck cancer patients (Table 4).
Several previous studies have reported that ANC is a prognostic marker of recurrence in head and neck cancer, among other cancers. Watanabe, et al. [20] reported that high ANC was associated with an increased risk of postoperative recurrence in intrahepatic cholangiocarcinoma. In addition, high pre-treatment ANC is a poor prognostic factor in uterine carcinoma and is associated with reduced progression-free survival [11]. The results of previous studies suggest that ANC is an inflammation-related marker. Wang, et al. [10] reported that abnormally high inflammatory cytokine levels affected the progression and recurrence of head and neck cancer. COX2 was activated by inflammation and exerted a protumorigenic effect through various mechanisms [21]. Higher COX2/P300 levels correlated with a higher recurrence rate in laryngeal squamous cell carcinoma [22]. The aforementioned studies suggest that inflammation is associated with the development and prognosis of cancer.
In our study, post-treatment ANC had a statistically significant positive correlation with the recurrence of head and neck cancer. In addition, the OS was decreased with high post-treatment ANC, although not statistically significantly. These findings are consistent with those of other studies that reported that graft-versus-host disease (GVHD) might cause tumorigenic inflammation. We propose that BMT followed by GVHD may create an inflammatory environment and tumorigenic effect. Cooke, et al. [23] suggested that GVHD reflects an abnormal immune regulatory mechanism that causes inflammation; this is supported by the higher ANC in GVHD patients compared to non-GVHD controls [24]. In our study, GVHD occurred in 12 (41%) of 16 (55%) patients after BMT, of whom 8 (28%) had head and neck cancer recurrence. This finding suggests that long-term oral cavity surveillance is necessary for patients who developed GVHD after BMT [25].
LDH is also used to predict cancer prognosis. High LDH level correlates with a poor prognosis in early stage and locally advanced cervical and colorectal cancers [26]. Although not statistically significant, we found that the OS was increased in patients with a high post-treatment LDH level (HR=7.256, 95% CI=0.906-58.120). Furthermore, the same molecular signaling cascade is involved in LDH metabolism and angiogenesis in tumors [27]. Azuma, et al. [28] reported that a high LDH serum level was associated with overexpression of the angiogenetic factors VEGFA and VEGFR-1. Tas, et al. [29] demonstrated that the LDH level was a surrogate marker of tumor angiogenesis. The previous reports agree with our conclusion that posttreatment LDH was negatively associated with OS.
Several hematological markers related to inflammation have been investigated in terms of their association with cancer. In particular, Gosavi and Torkadi [30] found that the serum C-reactive protein level was associated with oral squamous cell cancer and oral potentially malignant disorder, and showed a positive correlation with the primary tumor size. Many studies are being carried out on various hematologic markers that can be used to predict cancer prognosis. Several previous studies have investigated the use of various hematological markers to predict cancer prognosis. However, additional studies evaluating the molecular changes and imaging findings are needed to determine the clinical significance of hematological markers in cancers.
There were some limitations to our study. First, it was a retrospective study that included small numbers of patients in the various hematological malignancy categories, which limits the generalizability of our results. Second, the blood samples were collected at different times for each patient, which may have introduced bias into the results. Therefore, further large-scale, well-designed studies are required to verify our findings.
To the best of our knowledge, this is the first study to investigate the correlation between hematological markers and second primary head and neck cancer in patients with a previous hematological malignancy. The post-treatment ANC was negatively associated with the prognosis. ANC is related to inflammation, which is known to be associated with tumor pathogenesis. Our findings could aid the identification of patients requiring close follow-up after. However, further randomized controlled and prospective studies with large sample sizes are needed to validate our results.
ACKNOWLEDGMENTS
Statistical consultation was supported by the Department of Biostatistics of the Catholic Research Coordinating Center.
Notes
Author Contribution
Conceptualization: Sang-Yeon Kim, Dong-Il Sun, Jooin Bang. Data curation: Jooin Bang, Oh Hyeong Lee, Geun-Jeon Kim. Formal analysis: Jooin Bang, Geun-Jeon Kim. Investigation: Jooin Bang, Oh Hyeong Lee. Methodology: Jooin Bang. Software: Jooin Bang, Oh Hyeong Lee, Geun-Jeon Kim. Supervision: Dong-Il Sun, SangYeon Kim. Visualization: Jooin Bang. Writing—original draft: Jooin Bang. Writing—review & editing: Jooin Bang, Dong-Il Sun.
REFERENCES
1. Mowery A, Conlin M, Clayburgh D. Risk of head and neck cancer in patients with prior hematologic malignant tumors. JAMA Otolaryngol Head Neck Surg. 2019; 145(12):1121–7.

2. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021; 53(2):301–15.
3. Park WJ, Park JH, Cho S, Shin MG. Twenty-year incidence trend of hematologic malignancies in the Republic of Korea: 1999-2018. Blood Res. 2021; 56(4):301–14.

4. Hisada M, Chen BE, Jaffe ES, Travis LB. Second cancer incidence and cause-specific mortality among 3104 patients with hairy cell leukemia: A population-based study. J Natl Cancer Inst. 2007; 99(3):215–22.

5. Kumar V, Ailawadhi S, Bojanini L, Mehta A, Biswas S, Sher T, et al. Trends in the risk of second primary malignancies among survivors of chronic lymphocytic leukemia. Blood Cancer J. 2019; 9(10):75.

6. Feller A, Matthes KL, Bordoni A, Bouchardy C, Bulliard JL, Herrmann C, et al. The relative risk of second primary cancers in Switzerland: A population-based retrospective cohort study. BMC Cancer. 2020; 20(1):51.

7. Fitzthum AD, Wakely PE Jr. Chronic lymphocytic leukemia and second primary nonlymphoid malignancies: Cytopathologic study of 17 cases. J Am Soc Cytopathol. 2021; 10(3):321–7.

8. Ganzel C, Rowe JM. Prognostic factors in adult acute leukemia. Hematol Oncol Clin North Am. 2011; 25(6):1163–87.

9. Gorczyca W. Prognostic and predictive markers in hematologic neoplasms. A review. Pol J Pathol. 2011; 62(4):189–205.
10. Wang F, Arun P, Friedman J, Chen Z, Van Waes C. Current and potential inflammation targeted therapies in head and neck cancer. Curr Opin Pharmacol. 2009; 9(4):389–95.

11. Arend R, Van Arsdale A, Gojayev A, Roane BM, Doo D, Leath C, et al. Neutrophilia and mortality in women with uterine carcinosarcoma. Int J Gynecol Cancer. 2019; 29(8):1258–63.

12. Damar M, Dinç AE, Erdem D, Aydil U, Kizil Y, Eravcı FC, et al. Pretreatment neutrophil-lymphocyte ratio in salivary gland tumors is associated with malignancy. Otolaryngol Head Neck Surg. 2016; 155(6):988–96.

13. Koyama Y, Kawai S, Uenaka N, Okazaki M, Asaoka M, Teraoka S, et al. Absolute lymphocyte count, platelet-to-lymphocyte ratio, and overall survival in eribulin-treated HER2-negative metastatic breast cancer patients. Cancer Diagn Progn. 2021; 1(5):435–41.

14. Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013; 4(2):627–35.
15. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982; 143(1):29–36.

16. Lai CY, Tian L, Schisterman EF. Exact confidence interval estimation for the Youden index and its corresponding optimal cutpoint. Comput Stat Data Anal. 2012; 56(5):1103–14.

17. Dong C, Hemminki K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958-1996: A search for common mechanisms. Br J Cancer. 2001; 85(7):997–1005.

18. Laconi E, Marongiu F, DeGregori J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br J Cancer. 2020; 122(7):943–52.

19. Chen MH, Chang PM, Chen PM, Tzeng CH, Chu PY, Chang SY, et al. Prognostic significance of a pretreatment hematologic profile in patients with head and neck cancer. J Cancer Res Clin Oncol. 2009; 135(12):1783–90.

20. Watanabe A, Harimoto N, Araki K, Kubo N, Igarashi T, Tsukagoshi M, et al. Absolute neutrophil count predicts postoperative prognosis in mass-forming intrahepatic cholangiocarcinoma. Anticancer Res. 2019; 39(2):941–7.

21. Frejborg E, Salo T, Salem A. Role of cyclooxygenase-2 in head and neck tumorigenesis. Int J Mol Sci. 2020; 21(23):9246.

22. Chen YF, Luo RZ, Li Y, Cui BK, Song M, Yang AK, et al. High expression levels of COX-2 and P300 are associated with unfavorable survival in laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2013; 270(3):1009–17.

23. Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The biology of chronic graft-versus-host disease: A task force report from the National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017; 23(2):211–34.

24. Grkovic L, Baird K, Steinberg SM, Williams KM, Pulanic D, Cowen EW, et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012; 26(4):633–43.

25. Douglas CM, Jethwa AR, Hasan W, Liu A, Gilbert R, Goldstein D, et al. Long-term survival of head and neck squamous cell carcinoma after bone marrow transplant. Head Neck. 2020; 42(11):3389–95.

26. Wang H, Wang MS, Zhou YH, Shi JP, Wang WJ. Prognostic values of LDH and CRP in cervical cancer. Onco Targets Ther. 2020; 13:1255–63.
27. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2(1):38–47.
28. Azuma M, Shi M, Danenberg KD, Gardner H, Barrett C, Jacques CJ, et al. Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics. 2007; 8(12):1705–13.

Fig. 1.
Comparison of overall survival between head and neck cancer patients with and without previous hematological malignancy. H&N, head and neck.
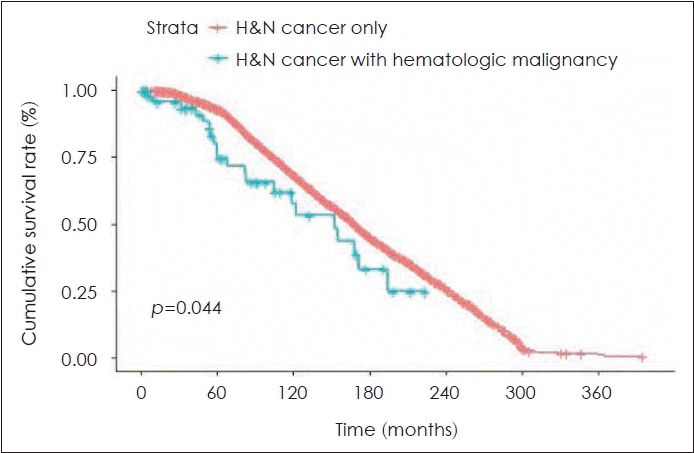
Fig. 2.
Receiver operating characteristic (ROC) curve of posttreatment absolute neutrophil count level.
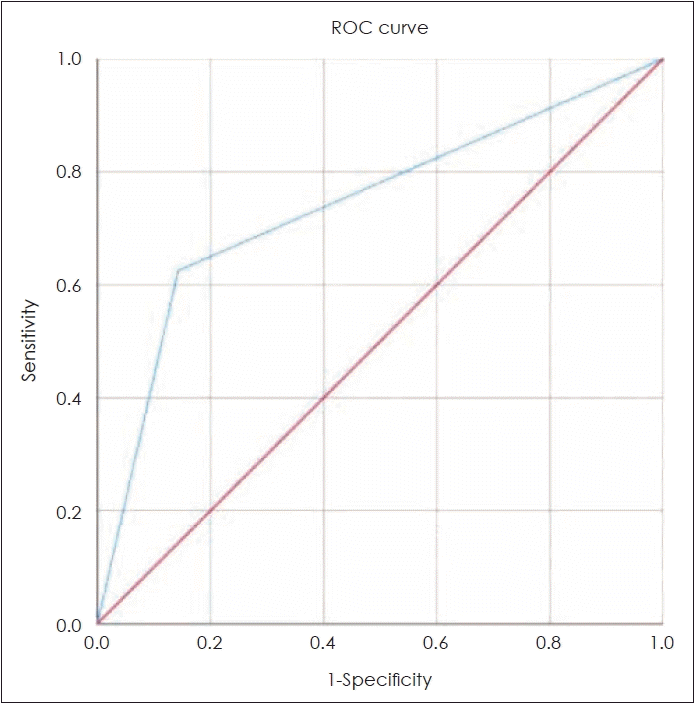
Table 1.
Baseline patient characteristics (n=29)
Table 2.
The serological parameters for the hematological malignancy subgroups
Table 3.
Univariate cox proportional hazard regression analysis of overall survival
Table 4.
Univariate Cox proportional hazard regression analysis of disease-free survival
| Parameters | HR (95% CI) | p value |
|---|---|---|
| LDH | ||
| Pre treatment | 0.549 | |
| ≥502 | 1.528 (0.381-6.125) | |
| <502 | Reference | |
| Post treatment | 0.061 | |
| ≥259 | 7.500 (0.909-61.847) | |
| <259 | Reference | |
| Absolute neutrophil count | ||
| Post treatment | 0.048* | |
| ≥6365 | 4.407 (1.046-18.566) | |
| <6365 | Reference | |
| Neutrophil-lymphocyte ratio | ||
| Post treatment | 0.122 | |
| ≥4.5 | 3.100 (0.739-13.011) | |
| <4.5 | Reference | |
| Platelet-lymphocyte ratio | ||
| Pre treatment | 0.334 | |
| ≥143 | 1.987 (0.494-80.02) | |
| <143 | Reference | |
| Post treatment | 0.536 | |
| ≥35 | 24.022 (0.001-5.644E5) | |
| <35 | Reference |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download