Abstract
Objective
Anterior cervical spine surgery (ACSS) is a common surgical procedure used to treat cervical spinal degenerative diseases. One of the complications associated with ACSS is prevertebral soft tissue swelling (PSTS), which can result in airway obstruction, dysphagia, and other adverse outcomes. This study aims to investigate the correlation between various cervical sagittal parameters and PSTS following single-level ACSS, as well as to identify independent risk factors for PSTS.
Methods
A retrospective study conducted at a single institution. The study population included all patients who underwent single-level ACSS between January 2014 and December 2022. Patients with a history of cervical spine surgery or trauma were excluded from the study. The presence and severity of PSTS was assessed by reviewing pre- and postoperative imaging studies. The potential risk factors for PSTS that were examined include patient age, sex, body mass index, tobacco use, comorbidities, serum albumin levels, operative time, implant type, implanted level, and various cervical spine sagittal parameters. Multivariate linear regression analysis was performed to identify the independent risk factors for PSTS.
Results
A total of 62 consecutive patients who underwent single-level ACSS over a 8-year period at a single institution were enrolled in this study. Only preoperative segmental angle showed positive correlation with PSTS among various cervical spine sagittal parameters (r=0.36, p=0.005). Artificial disc replacement showed a negative correlation with PSTS (β=-0.38, p=0.002), whereas the use of demineralized bone matrix (DBM) had a positive impact on PSTS (β=0.33, p=0.009). We found that male sex, lower preoperative serum albumin, and implantation of upper cervical level (above C5) were independent predictors for PSTS after single-level ACSS (β=1.21; 95% confidence interval [CI], 0.27 to 2.15; p=0.012; β=-1.63; 95% CI, -2.91 to -0.34; p=0.014; β=1.44; 95% CI, 0.38 to 2.49; p=0.008, respectively).
Conclusion
Our study identified male sex, lower preoperative serum albumin levels, and upper cervical level involvement as independent risk factors for PSTS after single-level ACSS. These findings can help clinicians monitor high-risk patients and take preventive measures to reduce complications. Further research with larger sample sizes and prospective designs is needed to validate these findings.
Prevertebral soft tissue swelling (PSTS) is a serious complication that can lead to airway obstruction following anterior cervical spine surgery (ACSS) [2,3,8,13,17,26]. Although the incidence of airway obstruction after ACSS is low at 5–6%, it can be fatal if it occurs, leading to respiratory failure and even brain death [20]. PSTS can also compress the pharynx or cervical esophagus, affecting epiglottic function, and contribute to dysphagia, dysphonia, and neck fullness after surgery [5,23].
Identifying risk factors for PSTS after ACSS can help clinicians monitor high-risk patients closely and prevent potential complications. Previous studies have identified operative time, number of segments operated, and level of surgery as significant risk factors for airway obstruction following ACSS [29]. However, existing literature on PSTS following ACSS has primarily focused on post-operative complications such as dysphagia and airway obstruction, with limited quantitative analysis of PSTS and related risk factors.
In our study, we conducted a retrospective analysis of patients who underwent single-level ACSS, quantitatively analyzing the degree of PSTS and exploring its potential correlation with cervical sagittal alignment parameters. The aim of our study was to identify independent risk factors associated with the degree of PSTS following single-level ACSS.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Hanyang University Guri Hospital (IRB; GURI 2023-04-011). Due to the retrospective nature of the study, the requirement for informed consent was waived by the IRB. Prior to analysis, all individual records were anonymized to protect patient privacy.
We conducted a retrospective analysis of electronic medical records of 192 patients who underwent ACSS at our hospital between January 2014 and December 2022. We included patients who had cervical spine radiographs taken both before and after the operation in our analysis to collect preoperative and postoperative radiological data. Patients diagnosed with symptomatic cervical degenerative disc disease with radiculopathy or myeloradiculopathy, within an age range of 19 to 80 were included in this study. Patients with a history of trauma, previous cervical spine surgery, or cerebral palsy were excluded from the study. In order to eliminate the confounding effect of multilevel surgery on PSTS, we included only patients who underwent single-level ACSS as study subjects. Finally, a total of 62 patients who had undergone single-level ACSS at our hospital were included in this study.
We examined various demographic parameters of the patients, including their age, sex, body mass index (BMI), comorbidities such as hypertension, diabetes mellitus, smoking status and alcohol consumption. We also took into account surgical procedural details such as operation time, amount of fluid input during surgery and involved cervical spinal segment. As a part of our analysis, we also looked into the preoperative and postoperative serum albumin levels of the patients to determine whether these levels had any correlation with the occurrence of PSTS after ACSS. All patients were monitored oxygen saturation from immediately after surgery until the removal of the drainage tube to assess the occurrence of respiratory distress. Additionally, the presence of symptoms related to soft tissue swelling, such as dyspnea, dysphagia, and dysphonia, was also monitored and confirmed during this period. Intravenous steroids were only administered for a short duration to patients who complained of dyspnea, dysphagia, dysphonia, or neck discomfort after the surgery. Patients who did not experience these symptoms did not routinely receive steroid treatment.
All surgical procedures were performed by one senior spinal neurosurgeon in our institute using a standard right SmithRobinson approach under general anesthesia [32]. After intubation, endotracheal tube cuff pressure was manually inflated to 20 mmHg and was not adjusted after retractor placement in all patients. A transverse skin incision was made, extending from the midline to the anterior border of the sternocleidomastoid muscle, in order to access the perpendicularly spreading fibers of the platysma muscle. The platysma muscle fibers were then split longitudinally. Total discectomy was performed at the index levels, involving the removal of the disc tissue, posterior longitudinal ligament, and bone spurs to ensure complete decompression. After thorough preparation of the end plates, a properly sized cervical cage or artificial disc was implanted. Layer-by-layer suturing and closure of the incision were performed, with the insertion of a drainage tube. According to the surgeon’s decision, the surgical procedures were broadly categorized into two methods : anterior cervical discectomy and fusion (ACDF) and artificial disc replacement (ADR). For ACDF, two different techniques were employed : standalone cage (SAC) and cage with plate fixation (CPF). In the ACDF surgeries, allograft cervical spacer or polyetheretherketone cage with demineralized bone matrix (DBM) were used. However, other bone graft substitutes such as bone morphogenetic protein-2 or synthetic peptides were not employed as part of the surgical procedure for any of the patients in this study. The cervical plate used in this study had consistent profile with a maximum thickness of 2.1 mm and a width of 16.9 mm. The plate was selected based on its design and compatibility with the surgical approach and fixation requirements. In the ADR surgeries, the same artificial disc product was consistently used for all patients.
Plain cervical spine lateral radiographs were taken in a standing position preoperatively, immediately postoperatively, and soft tissue neck lateral radiographs were taken daily for 5 days after the surgery. Using a PACS digital measuring instrument, we measured the anteroposterior (AP) thickness of the prevertebral soft tissue on the cervical spine lateral radiographs at each cervical level from C2 to C6, and the average values were calculated. The AP thickness of the prevertebral soft tissue was obtained by measuring the soft tissue space on a line parallel to the upper end plate, which extended from the midpoint of the anterior surface of each vertebral body to the border of the airway shadow (Fig. 1). This method allowed us to quantify and track the degree of PSTS daily for 5 days after surgery. The peak value of the difference between the preoperative and postoperative measurements was considered as the perioperative maximal PSTS after surgery.
In addition, the following cervical sagittal alignment parameters were also measured from cervical radiographs in the neutral position before and after surgery : O-C2 angle, C2-C7 angle, segmental angle, T1 slope, T1 slope minus cervical lordosis (T1s-CL), and cervical sagittal vertical axis (cSVA). The parameters used in this study were defined as follows : O-C2 angle was measured using the McRae line and the line tangential to the inferior aspect of the axis. C2-C7 angle was formed by the inferior aspect of the axis and C7. T1 slope was defined as the angle between a horizontal line and the upper end plate of T1. T1s-CL was calculated as the difference between the T1 slope and the C2-C7 angle. The cSVA was examined based on the horizontal offsets dropped by a vertical line from the mid-C2 vertebral body with respect to the mid-C7 vertebral body. Segmental angle was determined by measuring the Cobb angle between the upper endplate of the upper vertebral body and the lower endplate of the lower vertebral body at the surgical level (Fig. 2).
Two independent experienced neurosurgeons measured radiological parameters and the measurements were repeated by each observer after 2 weeks to evaluate inter- and intra-observer reliability using Pearson’s correlation analysis.
Descriptive statistics were used to summarize the demographic and clinical characteristics of the patients. Continuous variables were expressed as means±standard deviations (SDs), and categorical variables were presented as frequencies and percentages. Independent t-tests was employed for continuous variables, while chi-square tests were used for categorical variables, as appropriate. The mean differences in PSTS among operative levels were analyzed using the statistical method of analysis of variance (ANOVA). To analyze the differences in PSTS among different operative methods, we utilized ANOVA. Following ANOVA, we performed post hoc analysis using the Fisher’s least significant difference test to identify specific pairwise differences between the operative methods. The association between PSTS and various factors was assessed using univariate analysis. Factors found to be significant in the univariate analysis were further analyzed using multivariate linear regression analysis to determine their independent associations with PSTS. All statistical analyses were performed using SPSS software (version 28.0.0.0; IBM Corp., Armonk, NY, USA), and a p-value less than 0.05 was considered statistically significant.
The characteristics of the patients who underwent singlelevel ACSS are presented in Table 1. A total of 62 patients were included in this study, with 34 (54.8%) being male. The mean age of the patients was 73.2 years. The prevertebral soft tissue thickness before surgery had a mean value of 9.2 mm. After the ACSS procedure, the maximal prevertebral soft tissue thickness increased to a mean of 14.1 mm, indicating postoperative swelling. The perioperative maximal PSTS had a mean value of 5.0 mm, with a SD of 2.3.
Regarding the operative level distribution, the majority of patients had single-level ACSS performed at different levels. The differences in PSTS among each operative level were illustrated in Fig. 3. When the operative levels were classified into upper and lower cervical level based on C5 as the reference level, there was a statistically significant difference in PSTS between the two groups (p<0.001).
Among the 62 subjects included in the study, 41 patients underwent ACDF surgery with either an intervertebral spacer and anterior plate or stand-alone cage. Twenty-one patients underwent ADR surgery with the use of an artificial disc. The differences in PSTS among each operative method were illustrated in Fig. 4. In the post hoc analysis, there were significant differences in PSTS between ADR and SAC (p=0.014), as well as between ADR and CPF (p=0.005). However, there was no significant difference in PSTS between SAC and CPF (p=0.582).
Among the 62 patients included in the study, only four individuals experienced symptoms related to PSTS. Specifically, three patients complained of dysphagia, while the other one patient reported dysphonia. Among four patients with PSTS-related symptoms, the average PSTS was 6.99 mm.
Table 2 displays the mean values of each radiological parameter before and after the surgery, as well as the difference values (Δ) and p-values. The segmental angle exhibited a statistically significant increase of 1.7±5.5 degrees (p=0.019). The T1 slope showed an increase of 1.7±7.4 degrees, approaching statistical significance (p=0.069).
The variables that showed a significant association with PSTS in the univariate linear regression analysis were age (p=0.005), preoperative serum albumin (p=0.009), ADR surgery (p=0.002), DBM use (p=0.009), upper cervical level (p<0.001), a preoperative segmental angle (p=0.005). Hypertension and cervical plate fixation also showed a correlation with PSTS, although their p-values (0.071 and 0.069, respectively) were approaching statistical significance (Table 3). In the multivariate linear regression analysis, several variables were found to be significantly associated with PSTS. Male sex (1.212, p=0.012), lower preoperative serum albumin levels (-1.628, p=0.014), and upper cervical level (1.436, p=0.008) showed statistically significant standardized coefficients, indicating their independent contributions to the occurrence of PSTS. Radiological parameters and surgical technique related factors including operative method and DBM did not demonstrate statistically significant associations with PSTS (Table 4).
PSTS is a critical complication that can lead to airway obstruction, dysphagia, and dystonia after ACSS. Several studies have investigated the risk factors associated with complications such as dysphagia and airway obstruction following ACSS. Riley et al. [28] identified multilevel surgery and female sex as risk factors for dysphagia, while Sagi et al. [29], Emery et al. [7] reported that longer operation times and involvement of more than four segments, especially at the C4 or higher level, were risk factors for airway obstruction. However, the role of other factors such as age, gender, BMI, pathology, medical comorbidity, chronic obstructive pulmonary disease, and smoking remains unclear.
In our retrospective study, we focused specifically on patients who underwent single-level ACSS to investigate the risk factors associated with PSTS, which has not been extensively studied in this context. We conducted quantitative analyses of PSTS and cervical sagittal alignment parameters, which have not been thoroughly examined in previous studies. Our findings revealed some similarities, discrepancies, and completely different results compared to previous studies. Notably, our study excluded the confounding variable of multi-level involvement, providing a more focused analysis.
In the multivariate linear regression analysis, we found that male sex, lower preoperative serum albumin level, and upper cervical level involvement were statistically significant risk factors for PSTS. It is important to consider multiple variables in a clinical setting to obtain a comprehensive understanding, which necessitates conducting multivariate analysis. Although some variables that were statistically significant in univariate analysis lost their significance in multivariate analysis, this does not diminish their importance. These variables include age, radiological parameters, surgical technique related factors including operative method and DBM use.
Regarding sex, our findings contradicted previous studies that predominantly reported females as being more prone to PSTS [18,30,38]. Instead, our study revealed a higher incidence of PSTS in males, which aligns with the findings of Singh et al. [30] who reported a high incidence of dysphagia in males after ACSS involving fewer than three levels. These discrepancies can be resolved by expanding the sample size.
Low preoperative serum albumin levels were inversely correlated with PSTS, in line with the findings of Utariani et al. [37]. The hypoalbuminemic state is associated with prolonged inflammation, tissue edema, elevated levels of reactive oxygen species, muscle wasting, and increased mortality. These factors contribute to the occurrence of PSTS in patients with low preoperative serum albumin levels. Therefore, correcting albumin levels before surgery may help prevent PSTS.
Upper cervical level involvement, specifically at the C5 level and above, emerged as the most significant risk factor for PSTS. This finding is consistent with studies by Fujiwara et al. [11] which reported that all reintubated patients had fusion up to C3, and Suk et al. [36] which demonstrated that PSTS was significantly more severe in the proximal to C5 surgery group compared to the distal to C5 surgery group, with predominant swelling observed at the C2 and C3 levels. Andrew and Sidhu [1] also reported that the upper cervical spine has a greater potential retropharyngeal space compared to the lower cervical spine, which has a constrained anatomy and limited potential for swelling, further confirming our study’s findings.
Age was positively associated with PSTS, consistent with the findings of Singh et al. [30] that aging is an independent risk factor for PSTS and dysphagia after ACSS. Freemont and Hoyland [10] reported that in the elderly population, degenerative changes render musculoskeletal tissues less capable of adapting to soft tissue injury. Fragile bones, less resilient cartilage, weaker skeletal muscles, less elastic ligaments, and fat redistribution increase the likelihood of PSTS with advancing age.
Greater preoperative segmental angle, as measured by Cobb’s angle, was associated with increased PSTS. Anatomically, the cervical spine is lordotic, and our study revealed that a greater preoperative lordotic curve and greater segmental lordotic correction during surgery increase the risk of PSTS. This corresponds to the findings of Liu et al. [21] who showed that excessive cervical lordosis may lead to bulging of the posterior pharyngeal wall, reducing pharyngeal space and affecting normal pharyngeal squeeze and laryngeal elevation. Eun et al. [9] also demonstrated that excessive anterior and posterior segmental lordotic correction can cause stretching of the esophagus and compression of the posterior pharyngeal wall by the vertebra, leading to persistent dysphagia.
In the surgical technique, the group of patients who underwent ACDF had more prevertebral swelling compared to the group of ADR. Several studies have demonstrated that the group of patients undergoing ACDF had higher rates of prevertebral swelling and dysphagia compared to the group undergoing ADR [4,6,22,25,33]. Our results align with these findings. These differences can be attributed to factors such as surgical time, the extent of lordotic curve correction, and the use of plates, which will be mentioned in the following section. In our study, the use of DBM was found to be associated with an increased swelling. However, Other studies have reported that the use of DBM is not significantly associated with the occurrence of complications, such as swelling and dysphagia [12,16]. This discrepancy may be attributed to the differences in sample sizes between our study and previous research.
Although plate fixation has been extensively studied in previous research [15,18,19,21,27,31], our study found that it did not have a statistically significant association with PSTS in multivariate analysis. However, it is important to note that the plate fixation variable had a trend towards significance. Further research may be needed to elucidate the role of plate fixation in PSTS.
Indeed, endotracheal tube cuff pressure adjustment post-intubation is known to be a factor that could influence postoperative dysphagia. While there may still be some debate regarding its effect, it is essential to consider this aspect in research involving ACSS and PSTS [14,24,34,35]. All patients included in this study underwent uniform endotracheal tube management. Specifically, we maintained a consistent cuff pressure of 20 mmHg after retractor placement, without any subsequent adjustments throughout the surgical procedure. This standardized approach ensured that endotracheal cuff pressure did not act as a confounding factor in our investigation of PSTS-related symptoms.
Limitations of our study should be acknowledged in order to interpret the findings appropriately. Firstly, our study was conducted retrospectively, which inherently introduces limitations such as potential selection bias, incomplete data, and the inability to establish causal relationships. Conducting prospective studies would provide more robust evidence in this regard. Secondly, our study had a relatively small sample size of 62 patients, which might have limited the statistical power and the reliability of the results. Including a larger sample size would strengthen the study and increase the generalizability of the findings. Thirdly, we excluded cases with multi-level involvement to minimize confounding factors. However, this exclusion might restrict the generalizability of our findings to patients undergoing multi-level ACSS. To obtain a significant change in segmental angle, multi-level ACSS or posterior cervical spine surgery may be required. However, in this study, we focused exclusively on patients undergoing single-level ACSS. Consequently, the ability to establish a definitive causal relationship between cervical sagittal alignment parameters and PSTS is limited due to the restriction to single-level surgeries. To comprehensively investigate the association between cervical sagittal alignment and PSTS, additional research encompassing multi-level ACSS and posterior cervical spine surgeries is essential. Additionally, our study focused solely on identifying risk factors for postoperative PSTS immediately after ACSS. Including long-term follow-up data would provide valuable insights into the persistence, resolution, or recurrence of PSTS and its associated complications. Future studies with longer follow-up durations are warranted. Furthermore, while our study quantitatively analyzed PSTS itself as a risk factor, we did not include an analysis of complications such as dysphagia, airway obstruction, and dystonia. We mentioned the four patients who exhibited PSTS-related symptoms. However, due to the limited number of cases, establishing a definitive causal relationship between PSTS-related complications and specific factors was challenging. Investigating and analyzing these complications in relation to PSTS would further enhance our research.
In conclusion, our study provides valuable insights into the risk factors associated with PSTS in patients undergoing single-level ACSS. We observed that male sex, lower preoperative serum albumin level, and upper cervical level involvement were significant risk factors for PSTS. Furthermore, our study shed light on the influence of preoperative segmental angle on PSTS, emphasizing the need for careful consideration of the lordotic curve in surgical planning. Future studies with larger sample sizes are warranted to further investigate these risk factors and refine clinical practices related to PSTS and its complications.
Notes
Author contributions
Conceptualization : BJH, JIR; Data curation : SMK, YDW, MHH; Formal analysis : JP, BJH; Methodology : JP, YDW, MHH; Project administration : JHC, JIR; Visualization : JP, SMK, JIR; Writing - original draft : JP, BJH; Writing - review & editing : BJH, JIR
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korea Government (NRF-2021R1G1A1011491). The sponsor played no role in the study design, data collection, or analysis, or decision to submit the article for publication.
References
1. Andrew SA, Sidhu KS. Airway changes after anterior cervical discectomy and fusion. J Spinal Disord Tech. 20:577–581. 200.

2. Arimune M, Sanjou H, Yamada T, Yabe M, Miyake H. Minitracheotomy in treating upper airway obstruction after anterior cervical fusion. Masui. 53:1193–1196. 2004.
3. Benatar-Haserfaty J, Claros E. Respiratory failure from upper airway collapse following anterior cervical spine surgery. Rev Esp Anestesiol Reanim. 57:571–574. 2010.
4. Burkus JK, Haid RW, Traynelis VC, Mummaneni PV. Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: results from a prospective randomized controlled clinical trial. J Neurosurg Spine. 13:308–318. 2010.

5. Carucci LR, Turner MA, Yeatman CF. Dysphagia secondary to anterior cervical fusion: radiologic evaluation and findings in 74 patients. AJR Am J Roentgenol. 204:768–775. 2015.

6. Chang CJ, Liu YF, Hsiao YM, Huang YH, Liu KC, Lin RM, et al. Comparison of anterior cervical discectomy and fusion versus artificial disc replacement for cervical spondylotic myelopathy: a meta-analysis. J Neurosurg Spine. 37:569–578. 2022.

7. Emery SE, Smith MD, Bohlman HH. Upper-airway obstruction after multilevel cervical corpectomy for myelopathy. J Bone Joint Surg Am. 73:544–551. 1991.

8. Epstein NE, Hollingsworth R, Nardi D, Singer J. Can airway complications following multilevel anterior cervical surgery be avoided? J Neurosurg. 94(2 Suppl):185–188. 2001.

9. Eun DC, Suguitan AA, Suk KS, Kim HS, Kwon JW, Moon SH, et al. Variation in prevertebral soft tissue swelling after staged combined multilevel anterior-posterior complex cervical spine surgery: anterior then posterior (AP) versus posterior then anterior-posterior (PAP) surgery. J Clin Med. 11:7250. 2022.

10. Freemont AJ, Hoyland JA. Morphology, mechanisms and pathology of musculoskeletal ageing. J Pathol. 211:252–259. 2007.

11. Fujiwara H, Nakayama H, Takahashi H, Shimizu M, Hanaoka K. Postoperative respiratory disturbance after anterior cervical fusion. Masui. 47:475–478. 1998.
12. Han MS, Lee GJ, Kim JH, Lee SK, Moon BJ, Lee JK. Outcomes of anterior cervical fusion using polyetheretherketone cage with demineralized bone matrix and plate for management of subaxial cervical spine injuries. Korean J Neurotrauma. 14:123–128. 2018.

13. Harris OA, Runnels JB, Matz PG. Clinical factors associated with unexpected critical care management and prolonged hospitalization after elective cervical spine surgery. Crit Care Med. 29:1898–1902. 2001.

14. In ‘t Veld BA, Rettig TCD, de Heij N, de Vries J, Wolfs JFC, Arts MP. Maintaining endotracheal tube cuff pressure at 20 mmHg during anterior cervical spine surgery to prevent dysphagia: a double-blind randomized controlled trial. Eur Spine J. 28:353–361. 2019.

15. Kepler CK, Rihn JA, Bennett JD, Anderson DG, Vaccaro AR, Albert TJ, et al. Dysphagia and soft-tissue swelling after anterior cervical surgery: a radiographic analysis. Spine J. 12:639–644. 2012.

16. Kim SH, Lee JK, Jang JW, Park HW, Hur H. Polyetheretherketone cage with demineralized bone matrix can replace iliac crest autografts for anterior cervical discectomy and fusion in subaxial cervical spine injuries. J Korean Neurosurg Soc. 60:211–219. 2017.

17. Krnacik MJ, Heggeness MH. Severe angioedema causing airway obstruction after anterior cervical surgery. Spine (Phila Pa 1976). 22:2188–2190. 1997.

18. Lee MJ, Bazaz R, Furey CG, Yoo J. Influence of anterior cervical plate design on Dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech. 18:406–409. 2005.
19. Lee MJ, Bazaz R, Furey CG, Yoo J. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J. 7:141–147. 2007.

20. Lee SH, Kim KT, Suk KS, Park KJ, Oh KI. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion: a prospective, randomized study. Spine (Phila Pa 1976). 36:2286–2292. 2011.

21. Liu J, Hai Y, Kang N, Chen X, Zhang Y. Risk factors and preventative measures of early and persistent dysphagia after anterior cervical spine surgery: a systematic review. Eur Spine J. 27:1209–1218. 2018.

22. Loidolt T, Kurra S, Riew KD, Levi AD, Florman J, Lavelle WF. Comparison of adverse events between cervical disc arthroplasty and anterior cervical discectomy and fusion: a 10-year follow-up. Spine J. 21:253–264. 2021.

23. Martin RE, Neary MA, Diamant NE. Dysphagia following anterior cervical spine surgery. Dysphagia. 12:2–8. discussion 9-10. 1997.

24. Miller A, Griepp DW, Miller C, Hamad M, De la Garza Ramos R, Murthy SG. The effectiveness of reducing endotracheal cuff pressure after retractor placement to decrease postoperative laryngeal dysfunction in anterior cervical surgery: a meta-analysis. J Neurosurg Spine. 37:21–30. 2022.

25. Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 9:275–286. 2009.

26. Penberthy A, Roberts N. Recurrent acute upper airway obstruction after anterior cervical fusion. Anaesth Intensive Care. 26:305–307. 1998.

27. Riley LH 3rd, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976). 30:2564–2569. 2005.
28. Riley LH 3rd, Vaccaro AR, Dettori JR, Hashimoto R. Postoperative dysphagia in anterior cervical spine surgery. Spine (Phila Pa 1976). 35(9 Suppl):S76–S85. 2010.

29. Sagi HC, Beutler W, Carroll E, Connolly PJ. Airway complications associated with surgery on the anterior cervical spine. Spine (Phila Pa 1976). 27:949–953. 2002.

30. Singh K, Marquez-Lara A, Nandyala SV, Patel AA, Fineberg SJ. Incidence and risk factors for dysphagia after anterior cervical fusion. Spine (Phila Pa 1976). 38:1820–1825. 2013.

31. Smith-Hammond CA, New KC, Pietrobon R, Curtis DJ, Scharver CH, Turner DA. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine (Phila Pa 1976). 29:1441–1446. 2004.

32. Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 40:607–624. 1958.

33. Smucker JD, Bassuener SR, Sasso RC, Riew KD. Comparison of longterm differences in dysphagia: cervical arthroplasty and anterior cervical fusion. Clin Spine Surg. 30:E1160–E1164. 2017.
34. Sole ML, Su X, Talbert S, Penoyer DA, Kalita S, Jimenez E, et al. Evaluation of an intervention to maintain endotracheal tube cuff pressure within therapeutic range. Am J Crit Care. 20:109–117. quiz 118. 2011.

35. Sperry RJ, Johnson JO, Apfelbaum RI. Endotracheal tube cuff pressure increases significantly during anterior cervical fusion with the Caspar instrumentation system. Anesth Analg. 76:1318–1321. 1993.

36. Suk KS, Kim KT, Lee SH, Park SW. Prevertebral soft tissue swelling after anterior cervical discectomy and fusion with plate fixation. Int Orthop. 30:290–294. 2006.

37. Utariani A, Rahardjo E, Perdanakusuma DS. Effects of albumin infusion on serum levels of albumin, proinflammatory cytokines (TNF-α, IL-1, and IL-6), CRP, and MMP-8; tissue expression of EGRF, ERK1, ERK2, TGF-β, collagen, and MMP-8; and wound healing in sprague dawley rats. Int J Inflam. 2020:3254017. 2020.
Fig. 1.
The anteroposterior thickness of the prevertebral soft tissue was measured between the midpoint of the vertebral body’s anterior surface and the border of the airway shadow, as indicated by the arrow. A : Preoperative. B : Post-operative.

Fig. 2.
The O-C2 angle (O-C2A), the angle between McRae line and tangent line of axis’s inferior aspect; the C2-C7 angle (C2-7A), the angle between inferior aspects of axis and C7; the T1 slope, the angle between a horizontal line and the upper end plate of the T1 vertebra; the cervical sagittal vertical axis (cSVA), the distance between the horizontal offsets of the midpoints of C2 and C7; the segmental angle, the Cobb angle between the upper endplate of the upper vertebral body and the lower endplate of the lower vertebral body at the surgical level.
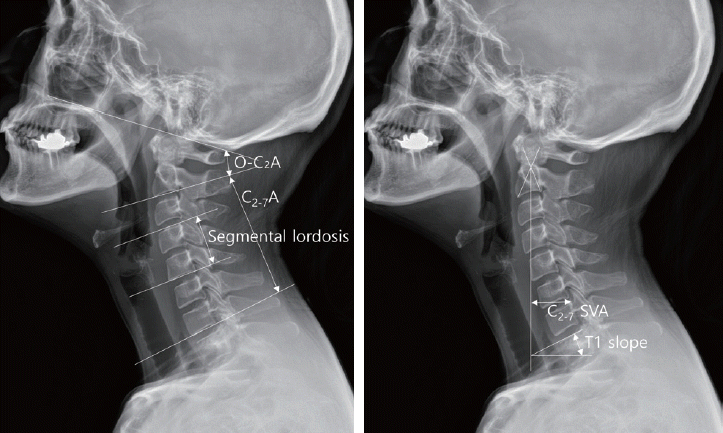
Fig. 3.
The illustration depicts the differences of mean prevertebral soft tissue swelling among each operative level.

Fig. 4.
The illustration depicts the differences of mean prevertebral soft tissue swelling among each operative method.
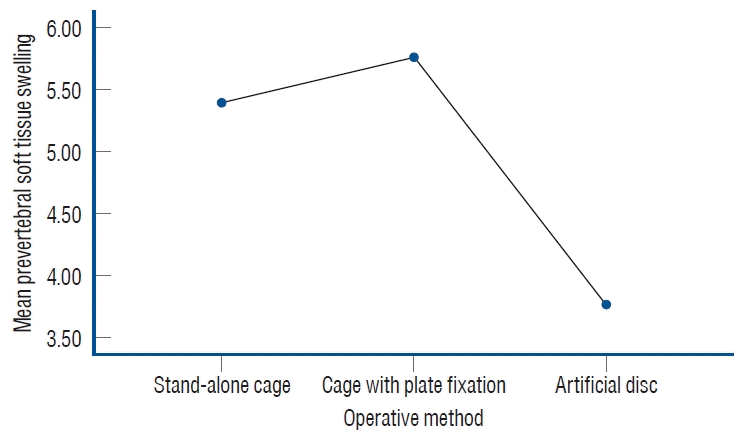
Table 1.
Patient demographics
Table 2.
Changes in radiological parameter values before and after single-level ACSS
| Variable | Pre-operation | Post-operation | Difference value (Δ) | p-value |
|---|---|---|---|---|
| O-C2 angle (°) | 17.3±7.8 | 16.5±6.8 | -0.8±7.0 | 0.354 |
| C2-C7 angle (°) | 13.1±7.4 | 13.9±8.3 | 0.8±7.5 | 0.414 |
| Segmental angle (°) | 4.0±4.4 | 5.7±4.4 | 1.7±5.5 | 0.019* |
| C2-C7 SVA (cm) | 23.9±10.4 | 24.3±9.7 | 0.4±13.7 | 0.801 |
| T1 slope (°) | 25.1±7.0 | 26.9±6.9 | 1.7±7.4 | 0.069 |
| T1s-CL (°) | 12.0±8.8 | 13.0±7.5 | 0.9±9.1 | 0.414 |
Table 3.
Univariate linear regression analysis of the association between prevertebral soft tissue swelling and various variables in patients undergone single-level ACSS
| Variable | Standardized beta |
95% CI |
p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Sex, male | 0.220 | -0.143 | 2.120 | 0.086 |
| Age | 0.352 | 0.021 | 0.114 | 0.005* |
| BMI | 0.098 | -0.074 | 0.167 | 0.447 |
| Preoperative serum albumin | -0.328 | -3.363 | -0.497 | 0.009* |
| Past medical history | ||||
| Hypertension | 0.231 | -0.092 | 2.197 | 0.071 |
| Diabetes | 0.127 | -1.064 | 3.141 | 0.327 |
| Smoking | 0.009 | -1.250 | 1.337 | 0.947 |
| Alcohol | 0.007 | -1.341 | 1.419 | 0.955 |
| Operative time | 0.081 | -0.021 | 0.041 | 0.531 |
| Fluid input during surgery | 0.036 | -0.002 | 0.003 | 0.783 |
| Plate fixation | 0.232 | -0.092 | 2.381 | 0.069 |
| Artificial disc replacement | -0.378 | -2.915 | -0.658 | 0.002* |
| DBM use | 0.327 | 0.375 | 2.574 | 0.009* |
| Upper cervical level, above C5 | 0.470 | 1.088 | 3.135 | <0.001* |
| Preoperative segmental angle | 0.355 | 0.058 | 0.307 | 0.005* |
| Preoperative T1 slope | -0.158 | -0.133 | 0.031 | 0.221 |
Table 4.
Multivariate linear regression analysis of the association between prevertebral soft tissue swelling and various variables in patients undergone single-level ACSS
| Variable |
Unstandardized coefficients |
Standardized coefficients (β) |
95% CI |
p-value | ||
|---|---|---|---|---|---|---|
| B | SE | Lower | Upper | |||
| Sex, male | 1.212 | 0.468 | 0.270 | 0.273 | 2.151 | 0.012* |
| Age | 0.001 | 0.025 | 0.006 | -0.049 | 0.052 | 0.961 |
| Preoperative serum albumin | -1.628 | 0.640 | -0.277 | -2.912 | -0.344 | 0.014* |
| Plate fixation | 0.399 | 0.600 | 0.081 | -0.805 | 1.603 | 0.509 |
| Artificial disc replacement | -0.113 | 0.911 | -0.024 | -1.939 | 1.713 | 0.902 |
| DBM use | 1.067 | 0.853 | 0.237 | -0.643 | 2.777 | 0.216 |
| Upper cervical level, above C5 | 1.436 | 0.525 | 0.320 | 0.384 | 2.488 | 0.008* |
| Preoperative segmental angle | 0.072 | 0.063 | 0.140 | -0.054 | 0.198 | 0.256 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download