Abstract
Objective
To analyze the outcomes of coil embolization (CE) for unruptured intracranial aneurysm (UIA) according to region and hospital size based on National Health Insurance Service data in South Korea.
Methods
The incidence of complications, including intracranial hemorrhage (ICRH) and cerebral infarction (CI), occurring within 3 months and the 1-year mortality rates in UIA patients who underwent CE in 2018 were analyzed. Hospitals were classified as tertiary referral general hospitals (TRGHs), general hospitals (GHs) or semigeneral hospitals (sGHs) according to their size, and the administrative districts of South Korea were divided into 15 regions.
Results
In 2018, 8425 (TRGHs, 4438; GHs, 3617; sGHs, 370) CEs were performed for UIAs. Complications occurred in 5.69% of patients seen at TRGHs, 13.48% at GHs, and 20.45% at sGHs. The complication rate in TRGHs was significantly lower than that in GHs (p=0.039) or sGHs (p=0.005), and that in GHs was significantly lower than that in sGHs (p=0.030). The mortality rates in TRGHs, GHs, and sGHs were 0.81%, 2.16%, and 3.92%, respectively, with no significant difference. Despite no significant difference in the mortality rates, the complication rate significantly increased as the number of CE procedures per hospital decreased (p=0.001; rho=-0.635). Among the hospitals where more than 30 CEs were performed for UIAs, the incidence of CIs (p=0.096, rho=-0.205) and the mortality rates (3 months, p=0.048, rho=-0.243; 1 year, p=0.009, rho=-0.315) significantly decreased as the number of CEs that were performed increased and no significant difference in the incidence of post-CE ICRH was observed.
With increases in the aging population, developments in diagnostic technology and improvements in the detection of unruptured intracranial aneurysms (UIAs), the demand for UIA treatment has increased [15]. In addition, as endovascular technology and device development have improved, the number of patients receiving endovascular treatment and the number of hospitals equipped for coil embolization (CE) continues to rise, which is a trend worldwide [15,18,20-22].
The incidence of hemorrhagic complications in stent-assisted CEs (SAC) ranges from 2.3% to 2.5% and from 1.9% to 2.7% in nonstent-assisted CE (NSAC). The incidence of ischemic complications after CE for UIAs ranged from 4.68% to 7.0% in SAC and from 2.0% to 3.5% in NSAC [4,24]. To explain these results, researchers have analyzed various factors affecting the outcomes of CE [1,3,11,24]. It has been reported that, aside from anatomic, technical, and patient factors, medical staff size, hospital size and region affect the clinical outcomes of CE [7,17,19].
In 2021, a study analyzing treatment outcomes in the entire Korean population with UIAs was published [14], and it was reported that the rate of complications was higher than that reported in other existing studies. It was hypothesized the quality of treatment decreased because of the rapid increase in the number of hospitals equipped for CE in recent years, and it was also hypothesized that the variation in treatment experience by region and hospital size also affected treatment quality [5,7,28].
Therefore, we analyzed the clinical outcomes in a nationwide cohort that underwent CE for UIAs by region and hospital size to identify the cause of the high incidence of complications.
This study was approved by the Institutional Review Board at Seoul National University Bundang Hospital (X-1810/498-903), and the requirement for informed consent was waived due to the retrospective nature of this study.
By using the National Health Insurance Service (NHIS) database, we were able to conduct a retrospective cohort study in which we assessed the treatment outcomes in patients who underwent CE for UIAs in 2018. Ninety-eight percent of Koreans are enrolled in the NHIS, and all clinical data, including examination, diagnosis, treatment, medical expenses, hospitalization and outpatient care, are stored in its database. Because the medical histories of 50 million single-race patients are provided by in the NHIS database, it is highly suitable for epidemiological and observational studies, as its reliability has already been validated in many studies [12,14-16]. Data were exclusively extracted from tertiary referral general hospitals (TRGHs), general hospitals (GHs), and semigeneral hospitals (sGHs), and data claimed from primary medical institutions were excluded to increase the reliability of the medical data.
Patients who underwent CE for UIAs were diagnosed according to the International Classification of Diseases (ICD), 10th revision diagnostic codes and then further classified by the Korean Classification of Diseases (KCD) codes. To extract the data concerning patients who underwent coil UIA embolization treatment in 2018, only the patients diagnosed with UIA (e.g., ICD code I67.1) were selected from among the patients who underwent CE (e.g., KCD codes M1661 and M1662) in 2018. Patients with a history of intracranial diseases such as subarachnoid hemorrhage, intracerebral hemorrhage, cerebral infarction (CI), brain trauma (e.g., ICD code S06-S09), or brain tumor (e.g., ICD code C41.0, C75.2, C71, C79.3, D32.9-D333, D35.3, or D44.4) before CE were excluded to accurately identify and classify patients with new complications such as an intracranial hemorrhage (ICRH) or CI that occurred after the procedure.
Hospitals were classified according to size as follows. A TRGH is a hospital with more than 500 beds, more than 20 departments, and specialists for each department. A GH is a hospital with more than 100 beds and more than seven departments, and an sGH is a hospital with more than 30 beds and more than seven departments. Regions were divided into 15 regions based on the administrative districts of the Republic of Korea, and hospitals in each region were analyzed by hospital size.
ICRHs, such as intracerebral hemorrhage, subarachnoid hemorrhage (SAH), subdural hematoma or epidural hematoma, and CI were analyzed to determine the incidence of postCE complications. First, we aimed to identify patients newly diagnosed with ICRHs (e.g., ICD code I60-I62) or CI (e.g., ICD code I63) within a 3-month period after CE procedure, despite having no prior history of these conditions. Then, we extracted relevant patient data meeting the specified criteria and further defined the cases as follows. To extract data on clinically significant ICRH, only the patients who underwent additional surgery, including hematoma drainage, craniotomy, or craniectomy (e.g., KCD codes N0321-N0324, S4621, S4622, and N0333), for ICRH were included in the analysis. Additionally, to identify patients with definite CI, excluding cases of subclinical CI, we only included those patients who had an inpatient stay of 5 days or more and underwent a magnetic resonance imaging examination during their hospitalization period, constituting the post-CE CI group.
Furthermore, we analyzed the all-cause mortality rates at 3 months and 1 year after CE to evaluate the short- and long-term outcomes of CE in patients who developed post-CE ICRH or CI. It was assumed that mortality due to causes other than post-CE ICRH or CI would be minimal because CE for UIA was performed on healthy patients with an expected life expectancy of more than 5 years, and mortality data were analyzed only for patients who experienced post-CE ICRH or CI.
Each outcome variable described above was analyzed according to the size of the hospitals in each region.
In order to verify the reliability of the study, a comparative analysis was performed using real-world data. The data from the institute that conducted the most CE procedures in 2018 was used for comparison with the corresponding NHIS data. The variables compared included the total number of patients who underwent CE for UIA in 2018, post-CE ICRH, post-CE CI, and mortality within 3 months. The institute with hospital-number 1 reported 623 patients, 0.80%, 0.64%, and 0.16% for each of these variables, respectively. The concordance rates between NHIS data and real-world data for each variable were found to be 98.2%, 81.9%, 98.8%, and 99.7%, respectively.
Data manipulation and extraction were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). The Wilcoxon signed rank test was performed to analyze the results according to the size of hospitals in each region, and pvalues <0.05 were considered significant. For the analysis of outcomes in patients receiving CE at each hospital, nonnormality was confirmed through the Kolmogorov-Smirnov test and the Shapiro-Wilk test, and then Spearman rank correlation analysis was performed. The rho value, which is a correlation coefficient, was derived using Spearman rank correlation analysis.
Nationwide, a total of 4497 CE procedures for UIAs were performed in 2013, while in 2018, the number rose to 8425, indicating an 87.3% increase over a 5-year period. SAC represented 42.2%, 53.6%, and 47.5% of all cases involving UIAs at TRGHs, GHs, and sGHs, respectively, in 2013. In contrast, the respective figures for SAC in 2018 were 55.6%, 59.4%, and 49.9% at TRGHs, GHs, and sGHs. Overall, the proportion of SAC procedures increased from 47.2% in 2013 to 57.0% in 2018, with a notable rise specifically observed at TRGHs. Figs. 1 and 2 depict the number of hospitals capable of performing CE by region in 2013 and 2018, as well as the number of CE procedures performed within 1 year.
Although the number of TRGHs available for CE procedures decreased from 43 in 2013 to 41 in 2018, the number of CE procedures for UIAs in TRGHs was 1.83 times larger, increasing from 2419 to 4438. To describe the four regions with the most TRGHs in descending order, Seoul had 13 TRHGs, Gyeonggi-do had five, Daegu had five and Busan had four. The number of CE procedures performed for UIAs was 2050 in Seoul, 787 in Gyeonggi-do, 464 in Daegu, and 365 in Busan. The number of GHs that could perform CE procedures was 222 in 2018, a 1.53-fold increase compared to 145 in 2013, and the number of CE procedures increased by 1.91-fold, from 1895 to 3617. Gyeonggi-do had 41 GHs, Seoul had 29, Busan had 18, and Gyeongsangnam-do had 18. The number of CEs performed for UIAs was 777 in Gyeonggi-do, 621 in Busan, 503 in Seoul, and 311 in Gyeongsangbuk-do. The number of sGHs available for CE was 248 in 2018, a 2.18-fold increase compared to 114 in 2013, and the number of CE procedures increased by 2.02-fold, from 183 to 370. The number of sGHs according to region was 55 in Gyeonggi-do, 28 in Busan, 25 in Gyeongsangnam-do, and 23 in Seoul. The number of CE procedures performed for UIAs in sGHs was 77 in Gyeonggi-do, 49 in Seoul, 37 in Busan, and 33 in Daegu. Overall, in 2018, there were 511 hospitals equipped for CE procedures, a 1.69-fold increase in the number of hospitals compared to 2013, and the total number of patients receiving CE was 8425, a 1.87-fold increase in patients treated over 5 years. In 2018, 101 hospitals were available for CE in Gyeonggi-do, 65 in Seoul, 50 in Busan, and 45 in Gyeongsangnam-do. The number of CE procedures performed for UIAs was 2602 in Seoul, 1641 in Gyeonggi-do, 1014 in Busan, and 519 in Daegu (Table 1).
Among the 8425 (TRGHs, 4438; GHs, 3617; sGHs, 370) patients who received CE for UIA in 2018, post-CE ICRH or CI complications occurred within 3 months in 5.69%, 13.48%, and 20.45% of patients seen at TRGHs, GHs, and sGHs, respectively. By comparing the size of hospitals nationwide, TRGHs had significantly lower complication rates than GHs (p=0.039) and sGHs (p=0.005), and GHs had significantly lower complication rates than sGHs (p=0.030). On the other hand, the 1-year mortality rates in patients seen at TRGHs, GHs, and sGHs were 0.81%, 2.16%, and 3.92%, respectively, and there was no significant difference when stratified by hospital size.
The complication rate in TRGHs was lowest in Gyeonggi-do (2.54%), followed by Gangwon-do (2.78%) and Seoul (3.12%). The complication rate in GHs was lowest in Gangwon-do (7.19%), followed by Gyeongsangnam-do (7.39%) and Ulsan (7.43%). The complication rate in sGHs was lowest in Jeollabuk-do (5.26%), followed by Seoul (10.20%) and Daejeon (13.33%) (Table 2).
Hospitals of the same size in the same region were integrated to compare the incidence of complications and mortality rates according to the number of CE procedures per hospital in 2018. The complication rate significantly increased as the number of CE procedures decreased (p=0.001; rho=-0.635). However, there was no significant difference in patient mortality rates within 1 year of the procedure when analyzed by the number of CE procedures per hospital (Table 3).
There was no significant difference in the complication or morality rate between hospitals of the same size in various regions in terms of the number of CEs performed (Table 4).
In 2018, there were 67 hospitals where more than 30 CE procedures for UIAs were performed, including 32 (78.0%) out of 41 TRGHs and 35 (14.9%) out of 222 GHs. The incidences of post-CE ICRH and CI in these hospitals were 1.8% and 6.5%, respectively, and the 6-month and 1-year mortality rates were 0.7% and 1.1%, respectively. There was no significant difference in the incidence of post-CE ICRH according to the number of CE procedures (p=0.096, rho=-0.205), but the CI incidence rate (p=0.005, rho=-0.340), 3-month mortality rate (p=0.048, rho=-0.243), and 1-year mortality rate (p=0.009, rho=-0.315) were significantly lower in hospitals that performed more CE procedures (Table 5).
After CE for UIAs, the incidence of ischemic complications, including thromboembolic events or CI, is known to range from 1.08% to 16.6%, and that of hemorrhagic complications, such as intraoperative rupture, has been reported to range from 0.0% to 9.5%. Additionally, the incidence of neurologic deficits due to procedure-related complications such as ischemic or hemorrhagic complications has been reported to be 0.27–14.7%, and the mortality rate is known to be 0.0–1.4% [2,6,8-10,13,23,25-27].
Most of the risk factors for complications that occur after CE procedures for intracranial aneurysms (IAs) are anatomical factors, technical failures (including device-related problems), and failure of antiplatelet treatments [1,3,11,24]. However, several studies have shown that the size of the hospital and the volume of CE procedures performed affect the treatment outcomes of IAs. Lindgren et al. [19] studied 8525 patients with aneurysmal SAHs (aSAHs) treated in TRGHs during 2007–2014 and analyzed the treatment results by classifying TRGHs into low (<41 procedures/year), intermediate (41–70 procedures/year), and high volume (>70 procedures/year) hospitals. They demonstrated that even within a subset of TRGHs, high hospital procedure volume (adjusted odds ratio, 0.56; 95% confidence interval, 0.36–0.87), which showed 5% crude 14-day case-fatality, is associated with lower case-fatality after aSAH regardless of treatment modality, thus supporting the relocation of patients to higher patient-volume centers [19]. In the United States, a study conducted using National Inpatient Sample (NIS) data from 2010–2011 showed better treatment outcomes of aSAHs in hospitals with a higher CE procedure volume, and a good prognosis was likely in hospitals where more than 35 CE procedures were performed per year [17]. In the United States, similar results can be seen in a study that analyzed the outcomes of CE for UIAs using NIS data from 1996–2000. They reported that CEs performed for UIAs at high-volume institutions (>24/year) or by high-volume physicians was associated with significantly lower morbidity rates and modestly lower mortality rates [7].
From 2009 to 2016, the incidence of a procedure-related CI after CE for UIA in Korea was 10.05%, and that of a hemorrhagic complication was 0.99%. As a result, the morbidity and mortality rates were 0.38% and 1.16%, respectively. Although this was an improvement in the clinical outcomes of CE for UIAs from 2006 to 2008 in Korea, the incidence of procedure-related CI in Korea was higher than that reported in previous literature [14]. As the results of this study show, from 2013 to 2018, the number of hospitals equipped for CE procedures increased by 1.69 times, of which GHs increased by 1.53 times and sGHs increased by 2.18 times. However, complications occurred in 5.69%, 13.48%, and 20.45% of patients treated at TRGHs, GHs, and sGHs, respectively, and as the size of the hospital decreased, the complication rate increased significantly even though there was no significant difference in the mortality rate. Overall, it was confirmed that the higher the number of CE procedures per hospital, the lower the complication rate.
For this reason, more patients with UIAs are being treated in central TRGHs, thus possibly compromising patient safety because of having to wait several months for UIA treatment. Urbanization has led to more patients being treated in urban hospitals and less patients being treated in rural hospitals, which makes it more difficult to improve the quality of medical care in rural hospitals, thus perpetuating a vicious cycle. Although it is advantageous for aSAH patients to receive rapid treatment at rural hospitals, more patients being treated in urban areas is likely to worsen the treatment outcomes of aSAH patients nationwide. To fundamentally solve these problems, policies targeting nationwide medical quality management and support are essential for the treatment of IAs.
There are some limitations to this study. Imaging information showing the location, size, and shape of UIA is not provided by the NHIS. Therefore, high-volume hospitals tend to treat more patients with UIAs, which is difficult to treat, so it was possible that there was no equal comparison between hospitals of different sizes. Because it was difficult to obtain accurate data on the patient’s neurological status, it was difficult to accurately assess the severity of complications, thus indicating a limitation in that the study indirectly evaluated complication severity using mortality. In addition, although there were differences in treatment results between hospitals of the same size in the same region, the accuracy of the analysis that was performed by integrating the size of hospitals in each region was lower. Nevertheless, this study is worthwhile in that it drew significant conclusions by using detailed operational definitions. Lastly, it should be acknowledged as a limitation of this medical research article that the analysis was based solely on data from the year 2018, without considering variables such as gender and age that could potentially influence treatment outcomes. However, considering the study’s objective of comparing treatment outcomes across various regions and hospital sizes, it was determined that utilizing data from a single year could still produce significant results. Furthermore, by conducting an analysis on the entire population of Korea rather than a sample group, we were able to partially address the aforementioned limitations.
Although there was no significant difference in the mortality rate, the complication rate of CE for UIA increased as the size of the hospital decreased and the volume of CE procedures per hospital decreased. To reduce the difference in the quality of cerebral aneurysm treatment by region and hospital size, nationwide medical quality management and support are needed.
Notes
ACKNOWLEDGMENTS
This study was supported by the Research Funds of the Korean Neuroendovascular Society.
References
1. Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke. 44:1348–1353. 2013.
2. Chien SC, Chen CC, Chen CT, Wang AY, Hsieh PC, Yeap MC, et al. Ticagrelor versus clopidogrel in stent-assisted coil embolization of unruptured intracranial aneurysms. Interv Neuroradiol. 28:568–574. 2022.

3. Choi HH, Cho YD, Yoo DH, Ahn SJ, Cho WS, Kang HS, et al. Stentassisted coil embolization of anterior communicating artery aneurysms: safety, effectiveness, and risk factors for procedural complications or recanalization. J Neurointerv Surg. 11:49–56. 2019.

4. Feng MT, Wen WL, Feng ZZ, Fang YB, Liu JM, Huang QH. Endovascular embolization of intracranial aneurysms: to use stent(s) or not? systematic review and meta-analysis. World Neurosurg. 93:271–278. 2016.

5. Gonda DD, Khalessi AA, McCutcheon BA, Marcus LP, Noorbakhsh A, Chen CC, et al. Long-term follow-up of unruptured intracranial aneurysms repaired in California. J Neurosurg. 120:1349–1357. 2014.

6. Higashi E, Matsumoto S, Nakahara I, Hatano T, Ishii A, Sadamasa N, et al. Clopidogrel response predicts thromboembolic events associated with coil embolization of unruptured intracranial aneurysms: a prospective cohort study. PLoS One. 16:e0249766. 2021.

7. Hoh BL, Rabinov JD, Pryor JC, Carter BS, Barker FG 2nd. In-hospital morbidity and mortality after endovascular treatment of unruptured intracranial aneurysms in the United States, 1996-2000: effect of hospital and physician volume. AJNR Am J Neuroradiol. 24:1409–1420. 2003.
8. Hwang G, Huh W, Lee JS, Villavicencio JB, Villamor RB Jr, Ahn SY, et al. Standard vs modified antiplatelet preparation for preventing thromboembolic events in patients with high on-treatment platelet reactivity undergoing coil embolization for an unruptured intracranial aneurysm: a randomized clinical trial. JAMA Neurol. 72:764–772. 2015.

9. Im SH, Han MH, Kwon OK, Kwon BJ, Kim SH, Kim JE, et al. Endovascular coil embolization of 435 small asymptomatic unruptured intracranial aneurysms: procedural morbidity and patient outcome. AJNR Am J Neuroradiol. 30:79–84. 2009.

10. Kabbasch C, Goertz L, Siebert E, Herzberg M, Borggrefe J, Mpotsaris A, et al. Comparison of WEB embolization and coiling in unruptured intracranial aneurysms: safety and efficacy based on a propensity score analysis. World Neurosurg. 126:e937–e943. 2019.

11. Kim B, Kim K, Jeon P, Kim S, Kim H, Byun H, et al. Thromboembolic complications in patients with clopidogrel resistance after coil embolization for unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 35:1786–1792. 2014.

12. Kim T, Lee H, Ahn S, Kwon OK, Bang JS, Hwang G, et al. Incidence and risk factors of intracranial aneurysm: a national cohort study in Korea. Int J Stroke. 11:917–927. 2016.

13. Kim YD, Kwon OK, Ban SP, Won YD, Bang JS, Kim T, et al. The inhibition rate estimated using VerifyNow can help to predict the thromboembolic risk of coil embolization for unruptured intracranial aneurysms. J Neurointerv Surg. 14:589–592. 2022.

14. Lee SH, Lee SU, Kwon OK, Bang JS, Ban SP, Kim T, et al. Clinical outcomes of clipping and coiling in elderly patients with unruptured cerebral aneurysms: a national cohort study in Korea. J Korean Med Sci. 36:e178. 2021.

15. Lee SU, Kim T, Kwon OK, Bang JS, Ban SP, Byoun HS, et al. Trends in the incidence and treatment of cerebrovascular diseases in Korea : part I. Intracranial aneurysm, intracerebral hemorrhage, and arteriovenous malformation. J Korean Neurosurg Soc. 63:56–68. 2020.

16. Lee SU, Kim T, Kwon OK, Bang JS, Ban SP, Byoun HS, et al. Trends in the incidence and treatment of cerebrovascular diseases in Korea : part II. Cerebral infarction, cerebral arterial stenosis, and moyamoya disease. J Korean Neurosurg Soc. 63:69–79. 2020.

17. Leifer D, Fonarow GC, Hellkamp A, Baker D, Hoh BL, Prabhakaran S, et al. Association between hospital volumes and clinical outcomes for patients with nontraumatic subarachnoid hemorrhage. J Am Heart Assoc. 10:e018373. 2021.

18. Lin N, Cahill KS, Frerichs KU, Friedlander RM, Claus EB. Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J Neurointerv Surg. 4:182–189. 2012.

19. Lindgren A, Burt S, Bragan Turner E, Meretoja A, Lee JM, Hemmen TM, et al. Hospital case-volume is associated with case-fatality after aneurysmal subarachnoid hemorrhage. Int J Stroke. 14:282–289. 2019.

20. Luther E, McCarthy DJ, Brunet MC, Sur S, Chen SH, Sheinberg D, et al. Treatment and diagnosis of cerebral aneurysms in the post-international subarachnoid aneurysm trial (ISAT) era: trends and outcomes. J Neurointerv Surg. 12:682–687. 2020.

21. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the international subarachnoid aneurysm trial (ISAT). Lancet. 385:691–697. 2015.

22. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): longterm follow-up. Lancet Neurol. 8:427–433. 2009.

23. Nii K, Inoue R, Morinaga Y, Mitsutake T, Hanada H. Evaluation of acute in-stent thrombosis during stent-assisted coil embolization of unruptured intracranial aneurysms. Neurol Med Chir (Tokyo). 58:435–441. 2018.

24. Nishido H, Piotin M, Bartolini B, Pistocchi S, Redjem H, Blanc R. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. AJNR Am J Neuroradiol. 35:339–344. 2014.

25. Pierot L, Spelle L, Vitry F; ATENA Investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 39:2497–2504. 2008.

26. Sedat J, Chau Y, Gaudart J, Sachet M, Beuil S, Lonjon M. Prasugrel versus clopidogrel in stent-assisted coil embolization of unruptured intracranial aneurysms. Interv Neuroradiol. 23:52–59. 2017.

Fig. 1.
Number of hospitals performing coil embolization for unruptured intracranial aneurysms, categorized by region and hospital size.
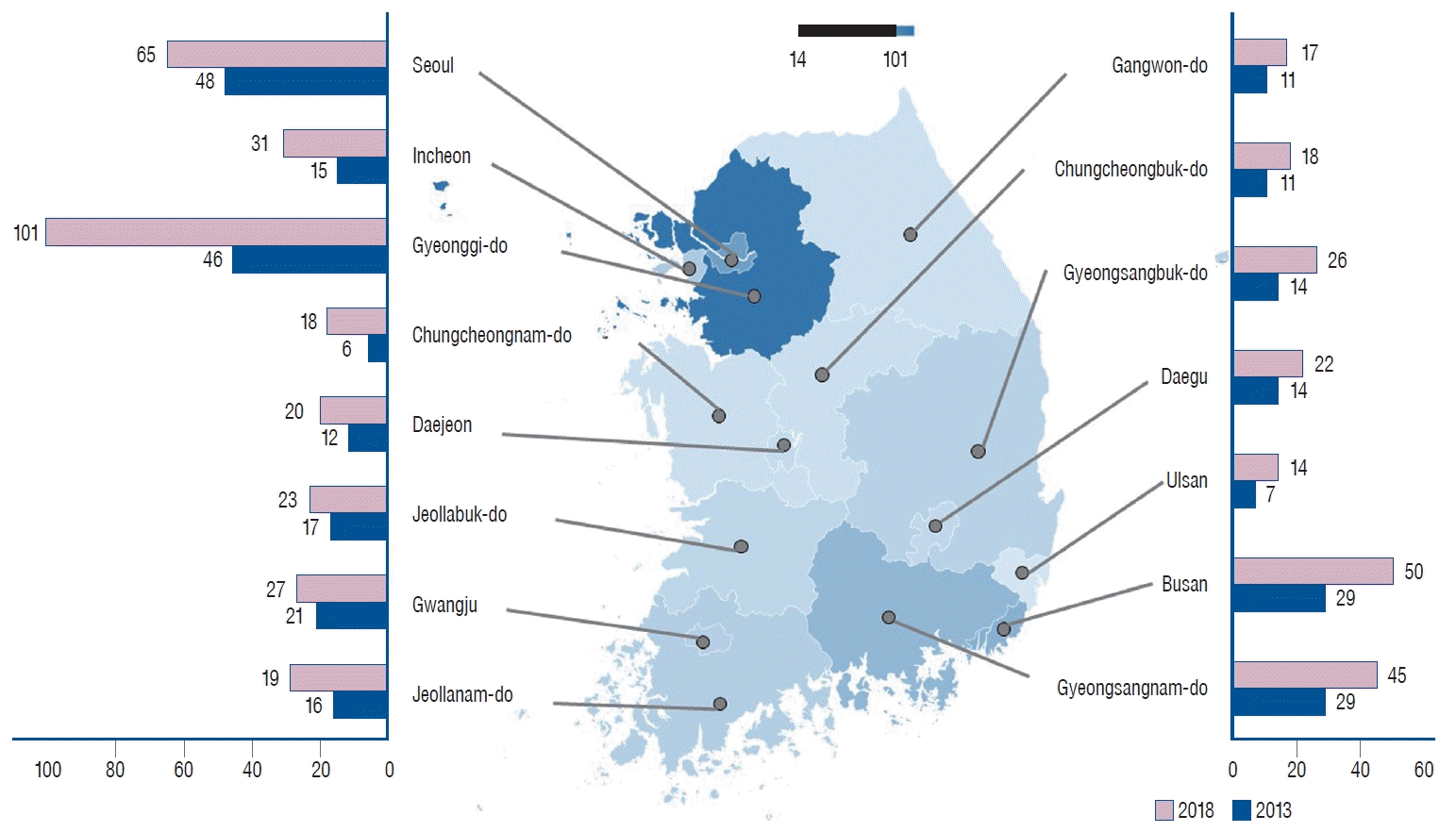
Fig. 2.
Number of coil embolization procedures for unruptured intracranial aneurysms, categorized by region and hospital size.
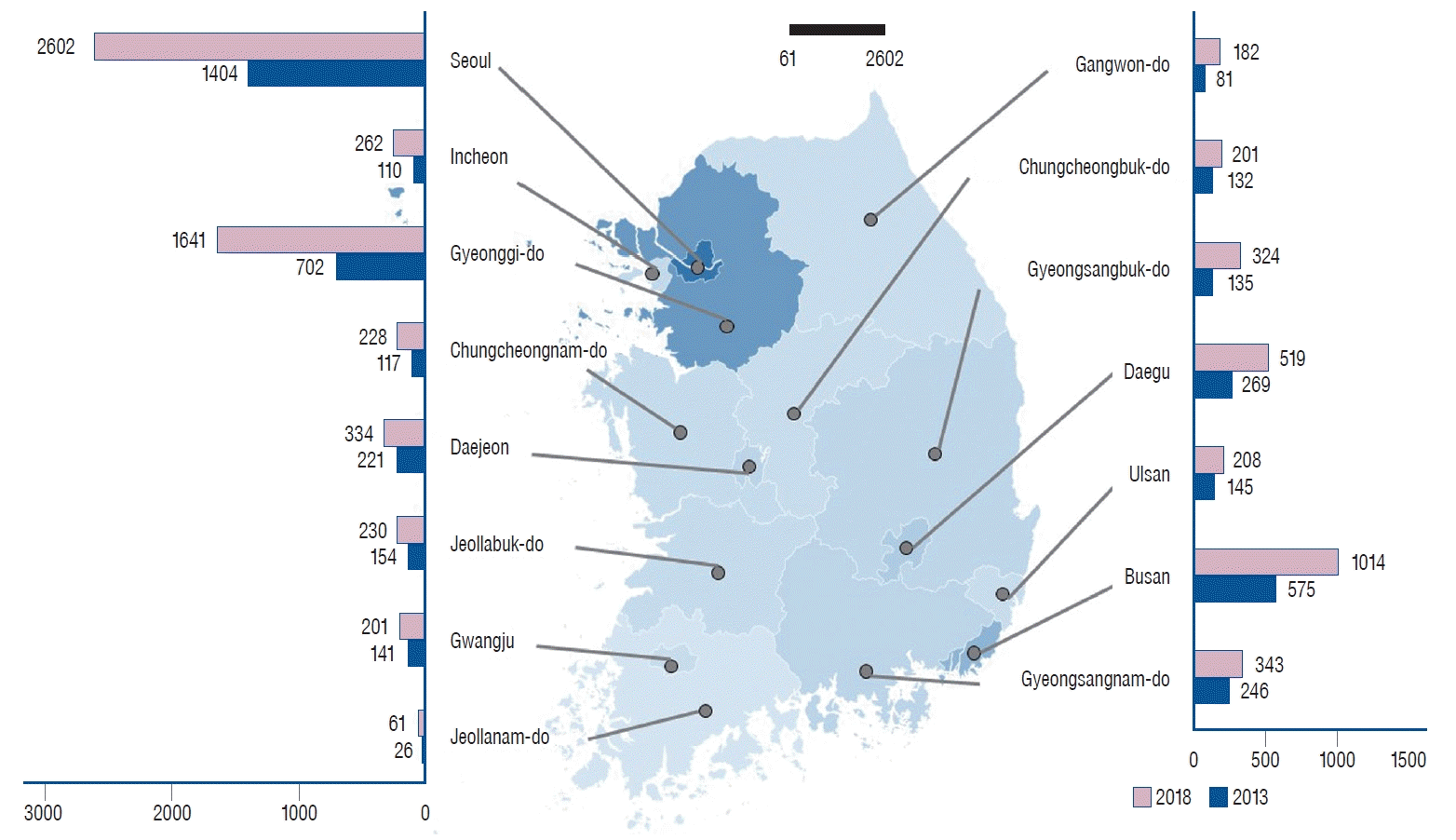
Table 1.
Number of hospitals allowing CE for UIAs, categorized by region and hospital size
Table 2.
Comparison of complications and mortality rates after CE for UIAs, categorized by region and hospital size
| TRGH (%) | GH (%) | p-value | TRGH (%) | sGH (%) | p-value | GH (%) | sGH (%) | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Complications rate within 3 months* | 0.039 | 0.005 | 0.030 | |||||||
| Overall | 5.69 | 13.48 | 5.69 | 20.45 | 13.48 | 20.45 | ||||
| Seoul | 3.12 | 13.92 | 3.12 | 10.20 | 13.92 | 10.20 | ||||
| Incheon | 11.46 | 9.09 | 11.46 | 17.65 | 9.09 | 17.65 | ||||
| Gyeonggi-do | 2.54 | 11.58 | 2.54 | 15.58 | 11.58 | 15.58 | ||||
| Gangwon-do | 2.78 | 7.19 | 2.78 | 14.29 | 7.19 | 14.29 | ||||
| Busan | 6.74 | 11.27 | 6.74 | 21.62 | 11.27 | 21.62 | ||||
| Daegu | 10.13 | 0.00 | 10.13 | 27.27 | 0.00 | 27.27 | ||||
| Ulsan | NA | 7.43 | NA | 33.33 | 7.43 | 33.33 | ||||
| Gyeongsangnam-do | 4.44 | 7.39 | 4.44 | 15.63 | 7.39 | 15.63 | ||||
| Gyeongsangbuk-do | NA | 12.22 | NA | 30.77 | 12.22 | 30.77 | ||||
| Gwangju | 10.24 | 11.48 | 10.24 | 15.38 | 11.48 | 15.38 | ||||
| Jeollanam-do | NA | 20.93 | NA | 16.67 | 20.93 | 16.67 | ||||
| Jeollabuk-do | 11.90 | 10.24 | 11.90 | 5.26 | 10.24 | 5.26 | ||||
| Daejeon | 5.26 | 12.37 | 5.26 | 13.33 | 12.37 | 13.33 | ||||
| Chungcheongnam-do | 6.58 | 9.15 | 6.58 | 20.00 | 9.15 | 20.00 | ||||
| Chungcheongbuk-do | 4.17 | 16.07 | 4.17 | 0.00 | 16.07 | 0.00 | ||||
| Mortality rate within 1 year | 0.753 | 0.937 | 0.975 | |||||||
| Overall | 0.81 | 2.16 | 0.81 | 3.92 | 2.16 | 3.92 | ||||
| Seoul | 0.34 | 1.99 | 0.34 | 0.00 | 1.99 | 0.00 | ||||
| Incheon | 0.64 | 1.14 | 0.64 | 23.53 | 1.14 | 23.53 | ||||
| Gyeonggi-do | 0.76 | 2.45 | 0.76 | 7.79 | 2.45 | 7.79 | ||||
| Gangwon-do | 2.78 | 2.16 | 2.78 | 0.00 | 2.16 | 0.00 | ||||
| Busan | 0.56 | 2.42 | 0.56 | 8.11 | 2.42 | 8.11 | ||||
| Daegu | 0.86 | 0.00 | 0.86 | 0.00 | 0.00 | 0.00 | ||||
| Ulsan | NA | 1.97 | NA | 16.67 | 1.97 | 16.67 | ||||
| Gyeongsangnam-do | 1.48 | 0.57 | 1.48 | 0.00 | 0.57 | 0.00 | ||||
| Gyeongsangbuk-do | NA | 1.61 | NA | 0.00 | 1.61 | 0.00 | ||||
| Gwangju | 1.57 | 0.00 | 1.57 | 0.00 | 0.00 | 0.00 | ||||
| Jeollanam-do | NA | 2.33 | NA | 0.00 | 2.33 | 0.00 | ||||
| Jeollabuk-do | 4.76 | 1.57 | 4.76 | 0.00 | 1.57 | 0.00 | ||||
| Daejeon | 4.51 | 2.15 | 4.51 | 0.00 | 2.15 | 0.00 | ||||
| Chungcheongnam-do | 1.32 | 2.11 | 1.32 | 0.00 | 2.11 | 0.00 | ||||
| Chungcheongbuk-do | 0.00 | 5.95 | 0.00 | 0.00 | 5.95 | 0.00 | ||||
Table 3.
Comparison of complications and mortality rates after CE for UIAs, categorized according to the number of CE procedures per hospital
| CE/hospital* (cases) | Complications rate† (%) | Mortality rate‡ (%) |
|---|---|---|
| 157.7 | 3.12 | 0.34 |
| 157.4 | 2.54 | 0.76 |
| 133.0 | 5.26 | 4.51 |
| 92.8 | 10.13 | 0.86 |
| 89.0 | 6.74 | 0.56 |
| 67.5 | 4.44 | 1.48 |
| 63.5 | 10.24 | 1.57 |
| 52.3 | 11.46 | 0.64 |
| 42.0 | 11.90 | 4.76 |
| 38.0 | 6.58 | 1.32 |
| 36.0 | 2.78 | 2.78 |
| 34.5 | 11.27 | 2.42 |
| 25.3 | 7.43 | 1.97 |
| 24.0 | 4.17 | 0.00 |
| 23.7 | 9.15 | 2.11 |
| 23.3 | 12.37 | 2.15 |
| 20.7 | 12.22 | 1.61 |
| 19.0 | 11.58 | 2.45 |
| 17.4 | 7.19 | 2.16 |
| 17.3 | 13.92 | 1.99 |
| 16.8 | 16.07 | 5.95 |
| 14.1 | 10.24 | 1.57 |
| 9.8 | 7.39 | 0.57 |
| 7.3 | 9.09 | 1.14 |
| 4.4 | 0.00 | 0.00 |
| 4.1 | 11.48 | 0.00 |
| 2.9 | 20.93 | 2.33 |
| 2.8 | 27.27 | 0.00 |
| 2.1 | 10.20 | 0.00 |
| 1.6 | 5.26 | 0.00 |
| 1.4 | 15.58 | 7.79 |
| 1.4 | 13.33 | 0.00 |
| 1.3 | 21.62 | 8.11 |
| 1.3 | 15.38 | 0.00 |
| 1.3 | 16.67 | 0.00 |
| 1.3 | 0.00 | 0.00 |
| 1.3 | 15.63 | 0.00 |
| 1.2 | 30.77 | 0.00 |
| 1.1 | 17.65 | 23.53 |
| 1.0 | 33.33 | 16.67 |
| 1.0 | 20.00 | 0.00 |
| 0.9 | 14.29 | 0.00 |
| p-value (rho) | 0.001 (-0.635) | 0.443 (-0.122) |
Table 4.
Complications and mortality rates after CE for UIAs, categorized according to the number of CE procedures per hospital
| Region |
Tertiary referral general hospital |
General hospital |
Semigeneral hospital |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coiling/hospital | Complications rate* (%) | p-value (rho) | Mortality rate† (%) | p-value (rho) | Coiling/hospital | Complications rate* (%) | p-value (rho) | Mortality rate† (%) | p-value (rho) | Coiling/hospital | Complications rate* (%) | p-value (rho) | Mortality rate† (%) | p-value (rho) | |
| Seoul | 157.7 | 3.12 | 0.542 (-0.196) | 0.34 | 0.665 (-0.140) | 17.3 | 13.92 | 0.871 (-0.044) | 1.99 | 0.104 -0.421 | 2.1 | 10.20 | 0.377 (-0.185) | 0.00 | 0.58 (-0.114) |
| Incheon | 52.3 | 11.46 | 0.542 (-0.196) | 0.64 | 0.665 (-0.140) | 7.3 | 9.09 | 0.871 (-0.044) | 1.14 | 0.104 -0.421 | 1.1 | 17.65 | 0.377 (-0.185) | 23.53 | 0.580 (-0.114) |
| Gyeonggi-do | 157.4 | 2.54 | 0.542 (-0.196) | 0.76 | 0.665 (-0.140) | 19.0 | 11.58 | 0.871 (-0.044) | 2.45 | 0.104 -0.421 | 1.4 | 15.58 | 0.377 (-0.185) | 7.79 | 0.580 (-0.114) |
| Gangwon-do | 36.0 | 2.78 | 0.542 (-0.196) | 2.78 | 0.665 (-0.140) | 17.4 | 7.19 | 0.871 (-0.044) | 2.16 | 0.104 -0.421 | 0.9 | 14.29 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Busan | 89.0 | 6.74 | 0.542 (-0.196) | 0.56 | 0.665 (-0.140) | 34.5 | 11.27 | 0.871 (-0.044) | 2.42 | 0.104 -0.421 | 1.3 | 21.62 | 0.377 (-0.185) | 8.11 | 0.580 (-0.114) |
| Daegu | 92.8 | 10.13 | 0.542 (-0.196) | 0.86 | 0.665 (-0.140) | 4.4 | 0.00 | 0.871 (-0.044) | 0.00 | 0.104 -0.421 | 2.8 | 27.27 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Ulsan | NA | NA | 0.542 (-0.196) | NA | 0.665 (-0.140) | 25.3 | 7.43 | 0.871 (-0.044) | 1.97 | 0.104 -0.421 | 1.0 | 33.33 | 0.377 (-0.185) | 16.67 | 0.580 (-0.114) |
| Gyeongsangnam-do | 67.5 | 4.44 | 0.542 (-0.196) | 1.48 | 0.665 (-0.140) | 9.8 | 7.39 | 0.871 (-0.044) | 0.57 | 0.104 -0.421 | 1.3 | 15.63 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Gyeongsangbuk-do | NA | NA | 0.542 (-0.196) | NA | 0.665 (-0.140) | 20.7 | 12.22 | 0.871 (-0.044) | 1.61 | 0.104 -0.421 | 1.2 | 30.77 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Gwangju | 63.5 | 10.2% | 0.542 (-0.196) | 1.57 | 0.665 (-0.140) | 4.1 | 11.48 | 0.871 (-0.044) | 0.00 | 0.104 -0.421 | 1.3 | 15.38 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Jeollanam-do | NA | NA | 0.542 (-0.196) | NA | 0.665 (-0.140) | 2.9 | 20.93 | 0.871 (-0.044) | 2.33 | 0.104 -0.421 | 1.3 | 16.67 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Jeollabuk-do | 42.0 | 11.90 | 0.542 (-0.196) | 4.76 | 0.665 (-0.140) | 14.1 | 10.24 | 0.871 (-0.044) | 1.57 | 0.104 -0.421 | 1.6 | 5.26 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Daejeon | 133.0 | 5.26 | 0.542 (-0.196) | 4.51 | 0.665 (-0.140) | 23.3 | 12.37 | 0.871 (-0.044) | 2.15 | 0.104 -0.421 | 1.4 | 13.33 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Chungcheongnam-do | 38.0 | 6.58 | 0.542 (-0.196) | 1.32 | 0.665 (-0.140) | 23.7 | 9.15 | 0.871 (-0.044) | 2.11 | 0.104 -0.421 | 1.0 | 20.00 | 0.377 (-0.185) | 0.00 | 0.580 (-0.114) |
| Chungcheongbuk-do | 24.0 | 4.17 | 0.542 (-0.196) | 0.00 | 0.665 (-0.140) | 16.8 | 16.07 | 0.871 (-0.044) | 5.95 | 0.104 -0.421 | 1.3 | 0.00 | 0.377 (-0.185) | 0.0 | 0.580 (-0.114) |
Table 5.
Patient clinical outcomes in hospitals where more than 30 CEs were performed for UIAs in 2018




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download