1. Strate LL, Morris AM. Epidemiology, pathophysiology, and treatment of diverticulitis. Gastroenterology. 2019; 156:1282–1298.

2. Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol. 2015; 110:1589–1596.

3. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016; 14:96–103.

4. Humes DJ, Solaymani-Dodaran M, Fleming KM, Simpson J, Spiller RC, West J. A population-based study of perforated diverticular disease incidence and associated mortality. Gastroenterology. 2009; 136:1198–1205.

5. Humes DJ, Fleming KM, Spiller RC, West J. Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut. 2011; 60:219–224.

6. Broersen LHA, Horváth-Puhó E, Pereira AM, Erichsen R, Dekkers OM, Sørensen HT. Corticosteroid use and mortality risk in patients with perforated colonic diverticular disease: a population-based cohort study. BMJ Open Gastroenterol. 2017; 4:e000136.

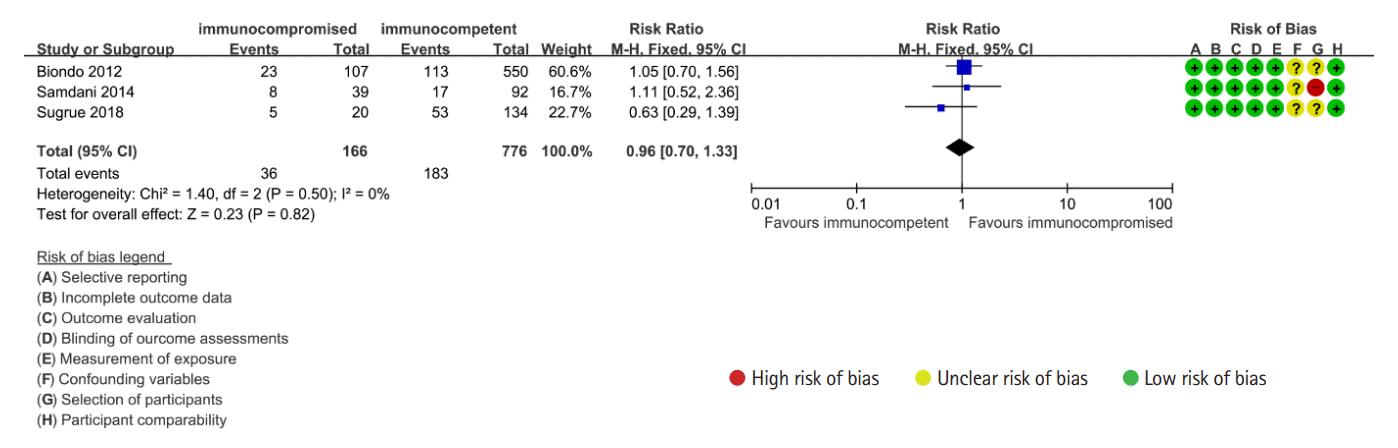
7. Biondo S, Borao JL, Kreisler E, et al. Recurrence and virulence of colonic diverticulitis in immunocompromised patients. Am J Surg. 2012; 204:172–179.

8. Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016; 59:254–261.

9. Tyau ES, Prystowsky JB, Joehl RJ, Nahrwold DL. Acute diverticulitis: a complicated problem in the immunocompromised patient. Arch Surg. 1991; 126:855–858.
10. Peery AF, Shaukat A, Strate LL. AGA clinical practice update on medical management of colonic diverticulitis: expert review. Gastroenterology. 2021; 160:906–911.

11. Nagata N, Ishii N, Manabe N, et al. Guidelines for colonic diverticular bleeding and colonic diverticulitis: Japan Gastroenterological Association. Digestion. 2019; 99 Suppl 1:1–26.

12. Sartelli M, Weber DG, Kluger Y, et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg. 2020; 15:32.
13. McKechnie T, Lee Y, Kruse C, et al. Operative management of colonic diverticular disease in the setting of immunosuppression: a systematic review and meta-analysis. Am J Surg. 2021; 221:72–85.

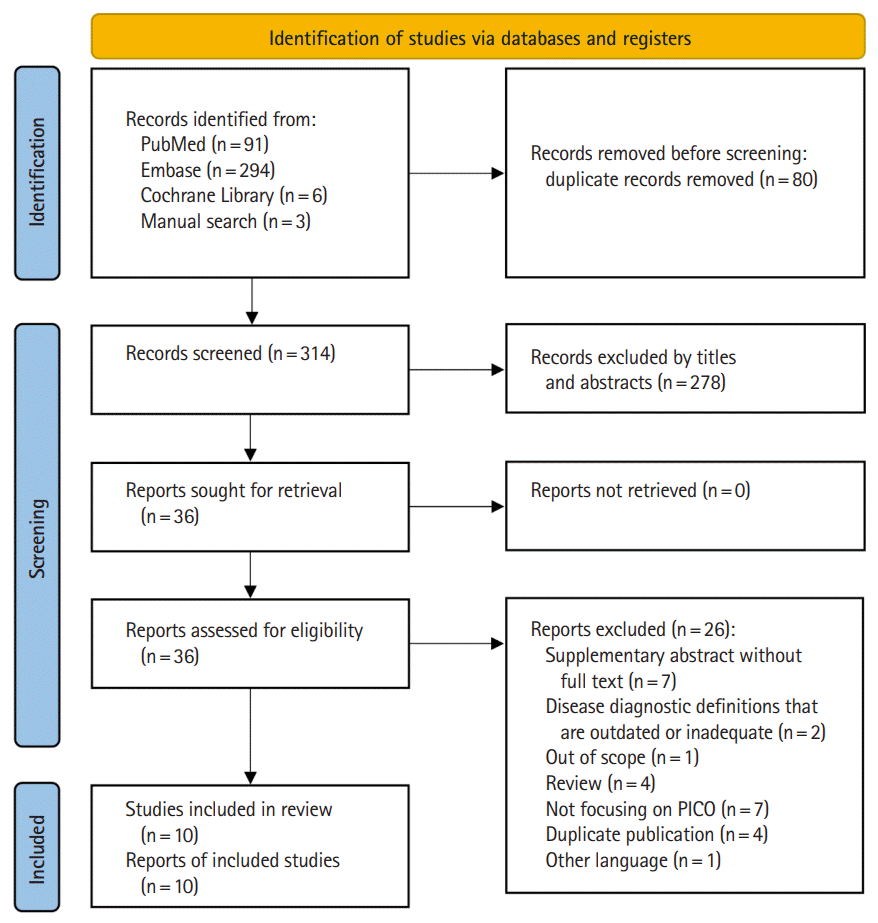
14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA Statement. BMJ. 2009; 339–b2535.
15. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66:408–414.

16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.

17. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022) [Internet]. c2022. [cited 2023 Mar 30].
www.training.cochrane.org/handbook.
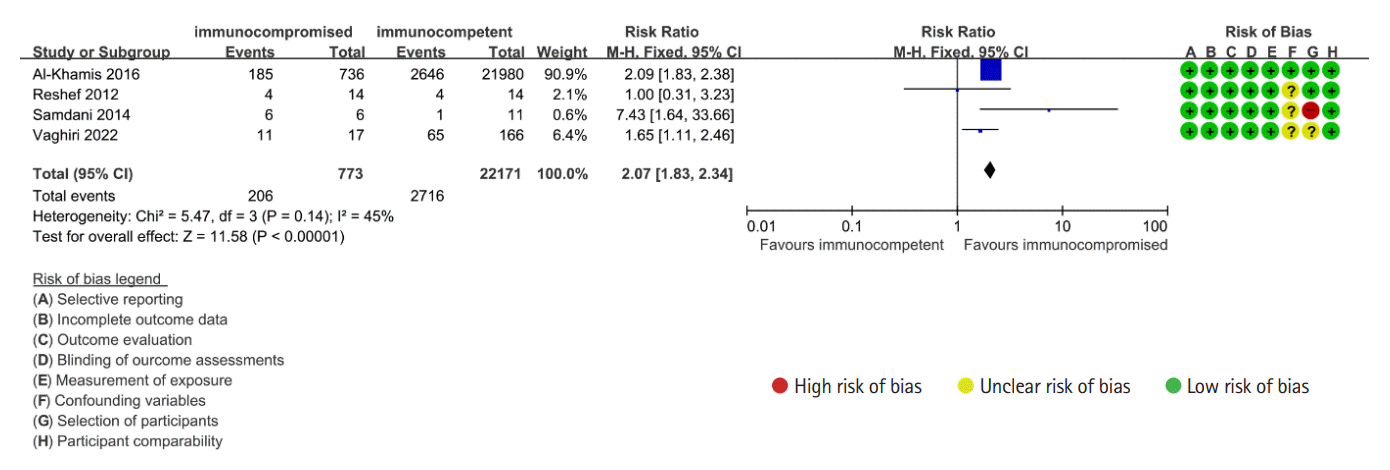
18. Reshef A, Stocchi L, Kiran RP, et al. Case-matched comparison of perioperative outcomes after surgical treatment of sigmoid diverticulitis in solid organ transplant recipients versus immunocompetent patients. Colorectal Dis. 2012; 14:1546–1552.

19. Cronley K, Wenzke J, Hussan H, et al. Diverticulitis in HIV-infected patients within the United States. HIV Med. 2016; 17:216–221.
20. Golda T, Kreisler E, Mercader C, Frago R, Trenti L, Biondo S. Emergency surgery for perforated diverticulitis in the immunosuppressed patient. Colorectal Dis. 2014; 16:723–731.

21. Al-Khamis A, Abou Khalil J, Demian M, et al. Sigmoid colectomy for acute diverticulitis in immunosuppressed vs immunocompetent patients: outcomes from the ACS-NSQIP database. Dis Colon Rectum. 2016; 59:101–109.

22. Samdani T, Pieracci FM, Eachempati SR, et al. Colonic diverticulitis in chemotherapy patients: should operative indications change? A retrospective cohort study Int J Surg. 2014; 12:1489–1494.

23. Halabi WJ, Jafari MD, Nguyen VQ, et al. Colorectal surgery in kidney transplant recipients: a decade of trends and outcomes in the United States. Am Surg. 2013; 79:1026–1033.

24. Vaghiri S, Prassas D, Knoefel WT, Krieg A. Surgical management in immunosuppressed patients with sigmoid diverticulitis, still a challenge: a single-center observational study. Int J Colorectal Dis. 2022; 37:1909–1917.

25. Sugrue J, Lee J, Warner C, et al. Acute diverticulitis in renal transplant patients: should we treat them differently? Surgery. 2018; 163:857–865.

26. Chabok A, Påhlman L, Hjern F, Haapaniemi S, Smedh K; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012; 99:532–539.
27. Daniels L, Ünlü Ç, de Korte N, et al. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017; 104:52–61.
28. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014; 57:284–294.

29. Hall J, Hardiman K, Lee S, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the treatment of left-sided colonic diverticulitis. Dis Colon Rectum. 2020; 63:728–747.

30. Francis NK, Sylla P, Abou-Khalil M, et al. EAES and SAGES 2018 consensus conference on acute diverticulitis management: evidence-based recommendations for clinical practice. Surg Endosc. 2019; 33:2726–2741.

31. Coccolini F, Improta M, Sartelli M, et al. Acute abdomen in the immunocompromised patient: WSES, SIS-E, WSIS, AAST, and GAIS guidelines. World J Emerg Surg. 2021; 16:40.

32. Bootun R. Effects of immunosuppressive therapy on wound healing. Int Wound J. 2013; 10:98–104.

33. Dean PG, Lund WJ, Larson TS, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004; 77:1555–1561.

34. Yamada E, Ohkubo H, Higurashi T, et al. Visceral obesity as a risk factor for left-sided diverticulitis in Japan: a multicenter retrospective study. Gut Liver. 2013; 7:532–538.

35. Kim MJ, Woo YS, Kim ER, et al. Is colonoscopy necessary after computed tomography diagnosis of acute diverticulitis? Intest Res. 2014; 12:221–228.

36. Oh HK, Han EC, Ha HK, et al. Surgical management of colonic diverticular disease: discrepancy between right- and left- sided diseases. World J Gastroenterol. 2014; 20:10115–10120.

37. Perkins JD, Shield CF 3rd, Chang FC, Farha GJ. Acute diverticulitis: comparison of treatment in immunocompromised and nonimmunocompromised patients. Am J Surg. 1984; 148:745–748.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download