Abstract
Objective
To systematically review the efficacy of e-Health interventions on physical performance, activity and quality of life in older adults with sarcopenia or frailty.
Methods
A systematic review was conducted by searching the MEDLINE, Embase, Cochrane Library, CINHAL, Web of Science, and the Physiotherapy Evidence Database for experimental studies published in English from 1990 to 2021. E-Health studies investigating physical activity, physical performance, quality of life, and activity of daily living assessment in adults aged ≥65 years with sarcopenia or frailty were selected.
Results
Among the 3,164 identified articles screened, a total of 4 studies complied with the inclusion criteria. The studies were heterogeneous by participant characteristics, type of e-Health intervention, and outcome measurement. Age criteria for participant selection and sex distribution were different between studies. Each study used different criteria for frailty, and no study used sarcopenia as a selection criteria. E-Health interventions were various across studies. Two studies used frailty status as an outcome measure and showed conflicting results. Muscle strength was assessed in 2 studies, and meta-analysis showed statistically significant improvement after intervention (standardized mean difference, 0.51; 95% confidence interval, 0.07–0.94; p=0.80, I2=0%).
Conclusion
This systematic review found insufficient evidence to support the efficacy of e-Health interventions. Nevertheless, the studies included in this review showed positive effects of e-Health interventions on improving muscle strength, physical activity, and quality of life in older adults with frailty.
Frailty and sarcopenia are critical topics in geriatric healthcare. These two conditions are major contributors to the decline in health and function in older adults. Frailty represents a vulnerability resulting from a decline in physical, cognitive, and social functioning, which makes it difficult to maintain healthy aging. Frailty is diagnosed as positive when three or more of the following five criteria are met: unintentional weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity level. This vulnerability increases the likelihood of hospitalization due to events such as infections, acute illnesses, and traffic accidents and prolongs recovery periods after acute illnesses [1].
Sarcopenia, a problem resulting from a loss of muscle mass and function, also impairs functional ability and reduces quality of life (QoL) in older adults. The European Working Group on Sarcopenia in Older People 2 defines sarcopenia primarily by low muscle strength in handgrip strength (cut-off points for male<27 kg and female<16 kg) and chair stand (cut-off points; >15 seconds for five rises). To confirm the diagnosis of sarcopenia, low muscle quantity or quality is required as measured by magnetic resonance imaging, computed tomography, or dual-energy X-ray absorptiometry. Severe sarcopenia is identified by concurrent low muscle strength, quantity/quality, and reduced physical performance, as assessed by tests such as the Short Physical Performance Battery, gait speed, the 400 m walk test, or the Timed Up and Go Test [2]. It makes it difficult for older adults to perform daily activities and increases the risk of mobility impairment and falls. Additionally, sarcopenia is associated with metabolic disorders, cardiovascular diseases, and Alzheimer’s disease, which increases the risk of developing such diseases [3].
Therefore, preventing and managing frailty and sarcopenia in geriatric healthcare is essential. This requires the use of various approaches, such as physical activity, nutrition, cognitive and social engagement, to maintain and improve older adults’ health and function. Regular health checkups are also necessary to continually manage older adults’ health [4].
The use of e-Health interventions in healthcare has become increasingly popular over the years. E-Health interventions refer to the use of digital technologies such as mobile apps, websites, wearable devices, and telemedicine to provide healthcare services remotely [5]. In the context of older adults with sarcopenia or frailty, e-Health interventions have the potential to address several challenges, including access to healthcare services, physical limitations, and social isolation [6]. However, older adults with sarcopenia or frailty may have difficulties participating in traditional exercise programs due to physical limitations, mobility issues, or a lack of access to resources. E-Health interventions can potentially address some of these barriers by providing personalized and adaptable exercise programs, remote monitoring and support, and social connections through virtual communities.
So, our study hypothesis is that e-Health interventions have the potential to increase physical activity and performance in older adults with sarcopenia or frailty. Previous studies suggest that e-Health could potentially improve rehabilitation outcomes for the elderly [7]. Various e-Health have shown a significant overall positive effect on strength and physical fitness in the elderly. However, the efficacy of e-Health on strength and physical fitness in the elderly with sarcopenia or frailty has not been established. This study aimed to systematically review the efficacy of e-Health interventions on physical performance, activity, and QoL in older adults with sarcopenia or frailty.
The protocol for this systematic review was registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO). The registration number is CRD42022315152. This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) statement guidelines. The completed PRISMA 2020 checklist is shown in Supplementary Materials S1 and S2.
(1) Participants (P): Aged population (over 65) with sarcopenia OR muscle atrophy OR frailty.
(2) Intervention (I): E-Health OR telemedicine OR web-based intervention.
(3) Comparison (C): No intervention.
(4) Outcomes (O): Main outcomes: physical activity, physical performance. Additional outcomes: health-related QoL, activity of daily living.
We included randomized controlled trials (RCTs) of the effects of e-Health on the elderly population (over 65) with sarcopenia, muscle atrophy, or frailty published in English. The types of interventions are e-Health, telemedicine, or web-based interventions. The types of comparators are no intervention. Exclusion criteria included: (1) full text is not available; (2) literatures includes diseases other than sarcopenia or frailty; (3) literatures does not include outcome parameters; and (4) duplicate publications.
We searched the MEDLINE, Embase, Cochrane Library, CINHAL, Web of Science, and the Physiotherapy Evidence Database (PEDro) for experimental studies published in English from 1990 to 2021, to obtain RCTs that studied the efficacy of e-Health for older adults with frailty or sarcopenia. The following key search terms were used: “Frailty,” “muscle atrophy,” and “sarcopenia.” The full search strategies, which were tailored according to the characteristics of the databases mentioned previously, are listed in Supplementary Material S3. We then manually searched the gray literature, reference lists of identified studies, MEDLINE, Embase, CINHAL, Web of Science, and PEDro for eligible RCTs.
Two reviewers independently identified eligible studies according to inclusion and exclusion criteria. After removing duplicates, primary selection was performed based on titles and abstracts. Then, titles, abstracts, and potentially relevant full texts were thoroughly reviewed according to eligible criteria by six reviewers, and any disagreements were resolved by discussion. (1) Study population must be geriatric patients (defined as above 65 years old). (2) Study must have implemented a method for randomization. (3) Study must include outcome measures of muscle mass or functional outcome.
Data extraction was performed in the inclusion study. Data were extracted by two reviewers, including study design, participants, intervention type, outcome measures, and results. A cross-check was performed to ensure no mistakes.
Two reviewers independently assessed the risk of bias of each included study by using the revised Cochrane risk of bias tool for individually randomized trials. Discrepancies were resolved through a team discussion.
We presented results as an effect with a standardized mean difference (SMD), and 95% confidence interval (95% CI) for continuous data and an odds ratio (OR), and 95% CI for binary data. Egger’s test was performed to check for publication bias. The heterogeneity across studies was assessed using Cochrane’s Q-test and I2-statistic. The random effects model was used for the meta-analysis. Meta-analysis was performed using Stata/MP version 16 (StataCorp LLC).
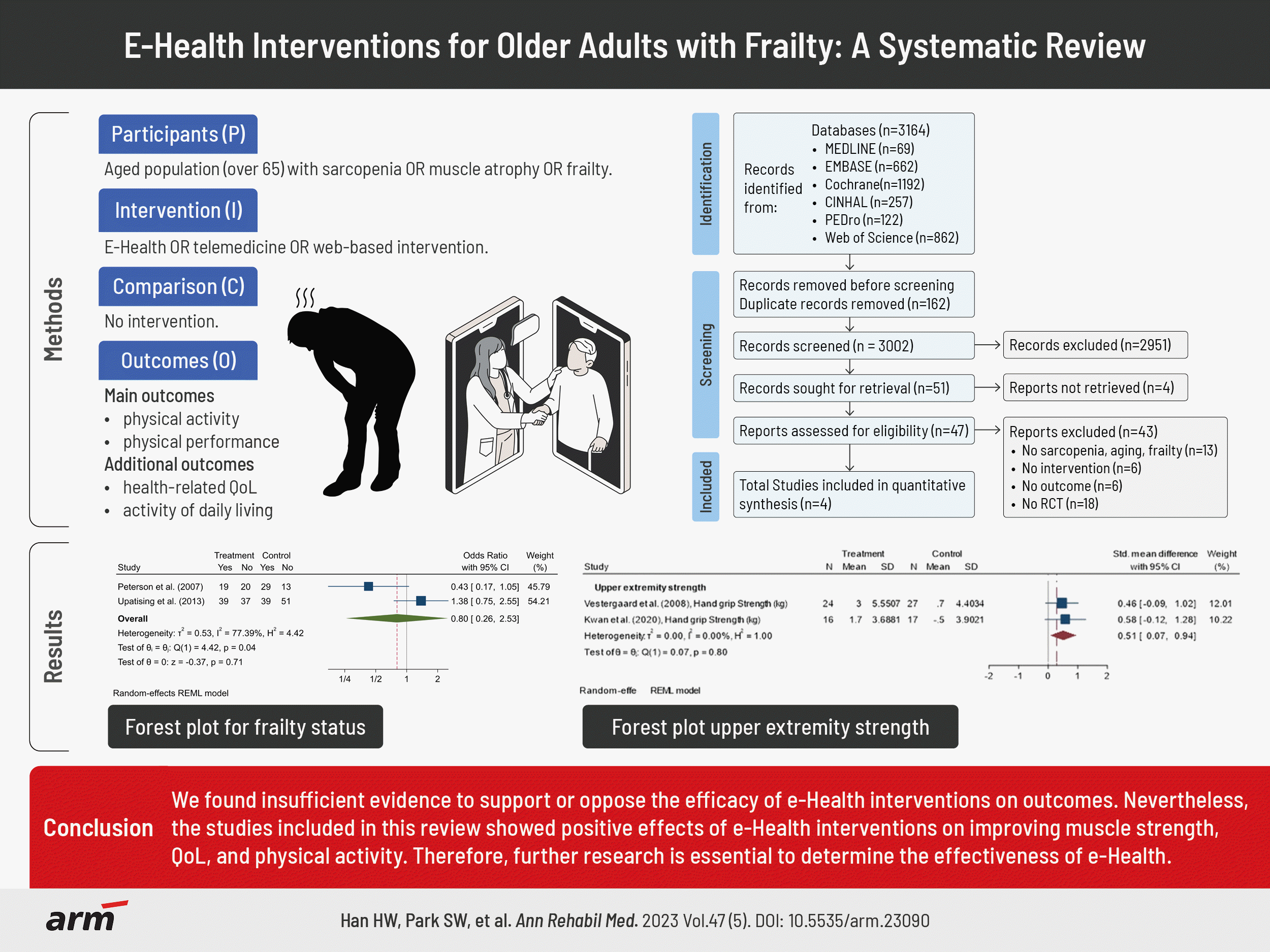
Overall, 3,164 publications were identified, and after the exclusion of duplicates, 3,002 articles remained. Of these, 2,951 were excluded, leaving 51 publications for potential inclusion. Of these, after applying the eligibility criteria, 47 were selected for full-text reading, after which a total of 4 articles were selected (Fig. 1). We could not find any studies related to sarcopenia, and studies related to frailty were included in this review.
In total, 380 paticipants were enrolled in the included studies, 187 in the intervention group (IG) and 193 in the control group (CG). Two studies recruited only male or female participants. Peterson et al. [8] recruited participants aged 70 and over (age, 78.4±4.9 years), and eighty-one elderly male veterans were randomized to intervention (n=39) or combined CGs (n=42). In the study of Vestergaard et al. [9], community-dwelling frail female≥75 years old, receiving public home care were randomized into a training group (n=30, age, 81.0±3.3 years) and a CG (n=31, age, 82.7±3.8 years). Other studies recruited both sexes. Upatising et al. [10] enrolled 205 adults aged 60 years or older (n=205, age, 80.4±8.3 years) with a high risk of hospitalization and emergency department visits (Elder Risk Assessment score of 16 or higher). Kwan et al. [11] recruited 33 adults aged ≥60 years (n=33, age, 71.0±9 years) with cognitive frailty and physical inactivity.
The e-Health interventions used in the selected articles were telephone exercise counseling, home-based video exercise intervention, telemonitoring case management, and mobile health (mhealth) intervention (i.e., smartphone-assisted programs using WhatsApp [Meta Platforms] and Samsung Health [Samsung Electronics]). The detailed interventions of each article are as follows. In the study of Peterson et al. [8], date, length, and specific exercise data for contact with the health care counselor were collected at the time of the call and recorded into the database. The health counselor followed up with phone calls once a month for the first three months and twice a week for the next three. Vestergaard et al. [9] provided all participants in the IG with a 30-minute video tape showing a booklet describing them, exercises, and an elastic resistance band. The IG exercised at home for 26 minutes, three times a week, for five months. In Upatising et al. [10], the telemonitoring process entailed installing the Intel® health guide, in addition to other peripheral devices, within the patient’s residence and establishing a connection to the healthcare system through a broadband network. The primary outcome at 6 months compared with baseline and at 12 months compared with 6 months. Kwan et al. [11] used a mobile phone application. Samsung Health is a physical activity autotracking app, and it continuously and autonomously monitors the walking patterns (e.g., steps, walking speed, walking time, physical activity intensity) of the users. The total duration of the intervention as a whole was 12 weeks.
According to the Cochrane risk of bias tool, all studies had a high risk of bias in the blinding of participants and personnel. One study had an unclear risk of bias in the allocation concealment. Half of the studies were had a risk of blinding of outcome assessment. All other bias categories had low risk of bias (Fig. 2).
Kwan et al. [11] examined physical activity with the Physical Activity Scale of Elderly and Moderate to Vigorous Physical Activity (MVPA) time (min/week) analysing 16 participants in the IG and 17 participants in the CG. In this study, MVPA time (median difference 86 min/week, p=0.04; median difference 18.5 min/valid day, p=0.02) significantly increased after the intervention in the IG only.
Vestergaard et al. [9] and Kwan et al. [11] examined physical performance with walking speed analysing 38 participants in the IG and 39 participants in the CG. In Vestergaard et al. [9], the IG significantly improved maximum walking speed by 8.2% (p=0.049), whereas the CG showed a significantly improved maximum walking speed of 7.4% (p=0.038).
Vestergaard et al. [9] and Kwan et al. [11] examined physical performance with hand grip strength (kg) analysing 40 participants in the IG and 43 participants in the CG. Within-group analysis showed significant improvements in handgrip strength by 17.1% in the IG, whereas no improvement was observed in the CG [9]. The forest plot showed that IG groups showed improvement after intervention, and no heterogeneity was noted (SMD, 0.51; 95% CI, 0.07–0.94; p=0.80, I2=0%; Fig. 3).
For both of the above outcomes, the publication bias could not be analyzed because there were two papers to be analyzed.
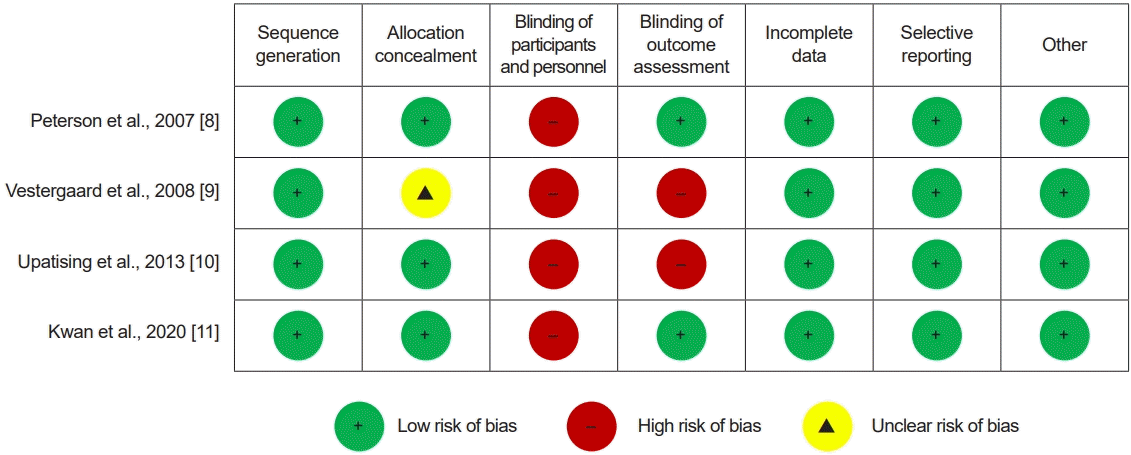
Peterson et al. [8] and Upatising et al. [10] examined frailty status with Fried frailty criteria analysing 68 participants in the IG and 81 participants in the CG. Peterson et al. [8] found that almost 70% of the participants were frail at baseline. In the IG, the proportion of frailty was reduced by 18% over 6 months, whereas there was no change in proportion over time in the CG. Upatising et al. [10] did not provide sufficient evidence to show that the telemonitoring group did better than usual care in decreasing the decline of frailty states and death. The forest plot showed that both groups had similar values (OR, 0.80; 95% CI, 0.26–2.53; p=0.04; I2=77.39%). A publication bias was observed (Egger’s test, p=0.035; Fig. 4).
Vestergaard et al. [9] examined QoL with EuroQol five-dimension scale questionnaire (EQ-5D), EQ visual analogue scale score, and S-R health analysing 24 participants in the IG and 27 participants in the CG. In this study, using an exercise video induced lasting health-related QoL (EQ-5D). In addition, improvements in physiological performance and functional capacity were generally observed [9].
In this systematic review and meta-analysis, we originally attempted to review RCTs of e-Health interventions for sarcopenia but could not find any. As a result, studies on frailty were included in the analysis, and we found insufficient evidence to support or oppose the efficacy of e-Health interventions on outcomes. Nevertheless, the studies included in this review showed positive effects of e-Health interventions on improving muscle strength, QoL, and physical activity in older adults with frailty or high risk. Two studies used frailty status as an outcome measure and showed conflicting results. Muscle strength was assessed in two studies, and a meta-analysis showed statistically significant improvement after intervention. Individual studies reported significant improvements in QoL and physical activity.
In another systematic review published recently, Esfandiari et al. [12] analyzed the effect of e-Health interventions on function and QoL for older adults with frailty. They analyzed data from 12 RCTs and found low evidence of benefits for telehealth interventions on function and the mental component of QoL. The effect of mHealth app interventions on sedentary time, physical activity and fitness in older adults (aged 55 years and older) was investigated in another systematic review by Yerrakalva et al. [13]. The review included six studies and found that mHealth app interventions may be effective in reducing sedentary time, increasing physical activity and improving fitness in the short term, but the results did not have a statistical significance. These reviews included more studies than ours due to different selection criteria, but their conclusions are consistent with ours.
The e-Health interventions used in the studies included in our review were various and not standardized. Earlier studies used only telephones [8] and videotapes [9] to encourage exercise. Upatising et al. [10] utilized broadband-based telemonitoring in addition to personal visits. Kwan et al. [11] introduced mobile technology to monitor and encourage exercise and give feedback. E-Health interventions can offer several strategies to encourage physical activity, including tailored exercise programs, virtual coaching and feedback, monitoring and tracking, social support, and gamification techniques, which have shown promise in improving physical activity levels in older adults. However, these advantages were not fully adopted in any of the studies included in this review [14,15]. This calls for future research.
The most notable e-Health intervention among the studies in this review is the one conducted by Kwan et al. [11] According to Kwan et al. [11], older adults’ levels of physical activity have increased by using mHealth activities. When considering the advantages of using a mobile phone over other forms of telerehabilitation, several key points emerge. Mobile phones have become ubiquitous, and a significant portion of the population owns smartphones and utilizes health-related applications. This widespread adoption of mobile technology makes it a convenient and accessible platform for delivering telerehabilitation services. And mobile phones offer a versatile and portable solution, enabling individuals to access therapy services anytime and anywhere, provided they have an internet connection. This flexibility promotes continuity of care and empowers patients to engage in rehabilitation exercises and activities conveniently. Moreover, mobile phones can support a range of multimedia capabilities, such as audio and visual communication, which are vital for effective telerehabilitation. Lastly, the use of mobile phones for telerehabilitation allows for personalized interactions and interventions through applications designed for healthcare professionals, medical students, patients, and the general public. These applications can provide tailored exercise programs, reminders, progress tracking, and educational resources, enhancing patient engagement and adherence to treatment plans. Their benefits, particularly in telerehabilitation, make them a powerful tool for delivering accessible, convenient, and personalized rehabilitation services.
The efficacy of e-Health in different populations has been reported by several studies. In one study, the effectiveness of a smartphone-based home care model for increasing the use of cardiac rehabilitation in myocardial infarction patients was evaluated. The study found that patients who used the smartphone-based home care model were more likely to complete their cardiac rehabilitation program and had better adherence to medication and lifestyle changes compared to those who received standard care. The study suggests that smartphone-based home care models may be a useful tool for improving the management of post-myocardial infarction patients [16]. Another study reported a pilot pragmatic RCT that evaluated how a mhealth app affected physical fitness and functional movement. Healthy male and female between the ages of 18 and 50 who could read and write English and who had a mobile phone that could download apps from the Apple App Store or Google Play Store were eligible to participate in the study. They were randomized to either an IG that utilized a mhealth app to track and monitor their physical activity or a CG that received standard care. According to the study, the IG significantly improved over the CG in functional movement and physical fitness. The authors conclude that the use of a mhealth app may be a useful tool for improving physical activity levels and functional movement in individuals [17]. Also, efficacy in a healthy geriatric population has been reported in several studies. Recent systematic reviews have demonstrated that eHealth interventions are successful at motivating elderly people (age>50 years) to exercise [18]. A healthy lifestyle is essential for reducing the prevalence of morbidity, functional restrictions, and impairment in older adults [19,20], increasing life expectancy, and enhancing overall QoL [21-23].
E-Health interventions are gaining more attention and importance, especially in the current post-pandemic era. A systematic review demonstrated the efficacy of telerehabilitation for patients experiencing disability due to coronavirus disease 2019 (COVID-19). The article’s key message was that physical activity should be given to those who have limited mobility as a result of isolation or lockdown in order to decrease their risk of developing frailty, sarcopenia, cognitive decline, and depression [24]. A clinical trial included in the systematic review introduced a telerehabilitation program in which the participants used either an internet-based platform or an application installed on their mobile phones. The platform allowed the physical therapist to adjust the number of sets, repetitions, speed, and observations for each patient. Therapeutic exercises were delivered in the form of an educational video with a detailed description. Through educational movies, the therapeutic education recommendations were explained to the patients, giving them health and emotional advice on how to improve their QoL after COVID-19. They reported that telerehabilitation led to a clinical improvement in QoL, particularly in the physical component [25]. These interventions can provide individuals with access to rehabilitation programs in the comfort of their own homes. E-Health interventions can be a useful alternative for improving frailty and sarcopenia in older adults, particularly during times of restricted mobility. In the future, the increased utilization of these interventions has the potential to enhance accessibility to rehabilitation programs for individuals in the convenience of their own homes, thereby improving their ability to maintain function and independence as they age. In one study [26], for hospitalized frail patients, the “VIVIFRAIL” multicomponent physical activity program was introduced [27]. This program consists of functional unsupervised exercises as well as supervised progressive resistance, balance, and walking exercises. Through the use of e-Health, patients can have access to such exercise programs and other healthcare services from the comfort of their homes, and the barriers to care for those who may have transportation or mobility issues or who live far away from specialists or clinics can be reduced. Additionally, e-Health can provide cost savings and convenience for both patients and healthcare providers [28]. By integrating exercise programs into e-Health platforms, patients can receive personalized exercise plans and have their progress monitored remotely by healthcare professionals, improving overall healthcare outcomes.
E-Health interventions can also be adopted for monitoring various aspects of patient information, such as nutrition and sleep quality, in addition to physical activities, for the purpose of improving frailty in older adults. E-Health can facilitate remote assessment and monitoring in multiple ways, provide personalized exercise programs, and offer virtual coaching and feedback [29]. The development of frailty in older persons is influenced by a number of significant modifiable factors, including nutrition [30]. And poor subjective sleep quality, various sleep symptoms, and longer sleep duration were found to increase the risk of frailty and even pre-frailty in an elderly population aged 70 to 84 years [31]. To manage these problems, an e-Health platform could provide information and resources on nutrition or sleep quality. One study showed a technique was developed and validated to assess pre-frailty risk in human activity patterns based on multimodal biomarkers, such as sleep duration, daily steps, and resting heart rate, collected from smartwatch sensors. specialized care. So, it could monitor sleep quality and provide feedback on how to improve sleep habits, which may help reduce the risk of frailty [32]. Additionally, researchers and practitioners can use motion sensors mounted on the upper limbs (i.e., lower arm/wrist, upper arm) to evaluate dietary intake and eating behavior in both laboratory and free-living conditions by using wearable sensing technology (e.g., commercial inertial sensors, fitness bands, and smart watches) [33]. To know what kind of food and how much to eat, there is a study that uses data from people wearing audio and motion sensors along with ground truth from continuous-scale video and data [34]. Another study effectively used a mobile phone image-based nutritional evaluation tool to help people with type 2 diabetes lose weight [35]. In frailty patients, for whom protein and calcium intake are important, monitoring these through e-Health seems to be of great help in nutritional management. However, to improve the adherence of e-Health programs in frailty patients, additional research is required to identify the optimal interventions as well as the best delivery methods and technologies [36].
Protecting the privacy and security of patient information is critical in the digital era of e-health. This is because unauthorized access and potential breaches in the virtual healthcare realm pose significantly higher risks. Cryptographic techniques, such as The Public Key Infrastructure, are essential to protect sensitive data. This method is additionally reinforced by the inclusion of biometric techniques. By utilizing distinctive individual traits like fingerprints or retina scans, biometric systems add a layer of security by guaranteeing that access is given only on the basis of physiological or behavioral characteristics. Additionally, it is essential to implement multi-factor authentication requiring several verifications prior to accessing the data and regular security audits. These thorough checks will ensure that the e-Health platforms are secure and safety [37,38].
There are several limitations in this systematic review. First, despite the growing interest in e-Health and telerehabilitation, there is still a lack of research on their effectiveness in improving frailty in older adults. Due to this limitation, only a few studies on this topic were included in our review. Second, the e-Health interventions in the four included studies were diverse and could not be standardized. More research is needed to better define effective e-Health measures for elderly population. Third, we found considerable heterogeneity among studies, stemming from diverse methodologies and outcomes, notably complicates our ability to draw cohesive conclusions. The specific criteria, settings, and population characteristics of these studies limit the generalizability of our findings to broader contexts. Thus, further research, emphasizing larger samples and consistent methodologies, is essential to advance our understanding.
Despite these limitations, our review suggests that e-Health may have a positive effect on frailty in older adults. Therefore, further research is necessary to determine the most effective e-Health interventions and understand their mechanisms of action. By addressing these issues, we can improve the quality of care for older adults with frailty or sarcopenia and promote healthy aging.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.5535/arm.23090.
Supplementary Material S3.
Detailed search strategies for online databases
REFERENCES
1. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–56.

2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31. Erratum in: Age Ageing 2019;48:601.

3. Hutchison ED. A life course perspective. In : Hutchison ED, editor. Dimensions of human behavior: the changing life course. Sage Publications;2010. p. 1–38.
4. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016; 31:3–10.

5. Hudson JL, Bower P, Kontopantelis E, Bee P, Archer J, Clarke R, et al. Impact of telephone delivered case-management on the effectiveness of collaborative care for depression and anti-depressant use: a systematic review and meta-regression. PLoS One. 2019; 14:e0217948.

6. Peek ST, Wouters EJ, van Hoof J, Luijkx KG, Boeije HR, Vrijhoef HJ. Factors influencing acceptance of technology for aging in place: a systematic review. Int J Med Inform. 2014; 83:235–48.

7. Núñez de Arenas-Arroyo S, Cavero-Redondo I, Alvarez-Bueno C, Sequí-Domínguez I, Reina-Gutiérrez S, Martínez-Vizcaíno V. Effect of eHealth to increase physical activity in healthy adults over 55 years: a systematic review and meta-analysis. Scand J Med Sci Sports. 2021; 31:776–89.

8. Peterson MJ, Sloane R, Cohen HJ, Crowley GM, Pieper CF, Morey MC. Effect of telephone exercise counseling on frailty in older veterans: project LIFE. Am J Mens Health. 2007; 1:326–34.

9. Vestergaard S, Kronborg C, Puggaard L. Home-based video exercise intervention for community-dwelling frail older women: a randomized controlled trial. Aging Clin Exp Res. 2008; 20:479–86.

10. Upatising B, Hanson GJ, Kim YL, Cha SS, Yih Y, Takahashi PY. Effects of home telemonitoring on transitions between frailty states and death for older adults: a randomized controlled trial. Int J Gen Med. 2013; 6:145–51.

11. Kwan RY, Lee D, Lee PH, Tse M, Cheung DS, Thiamwong L, et al. Effects of an mHealth brisk walking intervention on increasing physical activity in older people with cognitive frailty: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2020; 8:e16596.

12. Esfandiari E, Miller WC, Ashe MC. The effect of telehealth interventions on function and quality of life for older adults with pre-frailty or frailty: a systematic review and meta-analysis. J Appl Gerontol. 2021; 40:1649–58.

13. Yerrakalva D, Yerrakalva D, Hajna S, Griffin S. Effects of mobile health app interventions on sedentary time, physical activity, and fitness in older adults: systematic review and meta-analysis. J Med Internet Res. 2019; 21:e14343.

14. Forman CR, Nielsen JB, Lorentzen J. Neuroplasticity at home: improving home-based motor learning through technological solutions. A review. Front Rehabil Sci. 2021; 2:789165.

15. Laver K, George S, Ratcliffe J, Quinn S, Whitehead C, Davies O, et al. Use of an interactive video gaming program compared with conventional physiotherapy for hospitalised older adults: a feasibility trial. Disabil Rehabil. 2012; 34:1802–8.

16. Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014; 100:1770–9.

17. Stork MJ, Bell EG, Jung ME. Examining the impact of a mobile health app on functional movement and physical fitness: pilot pragmatic randomized controlled trial. JMIR Mhealth Uhealth. 2021; 9:e24076.
18. Elavsky S, Knapova L, Klocek A, Smahel D. Mobile health interventions for physical activity, sedentary behavior, and sleep in adults aged 50 years and older: a systematic literature review. J Aging Phys Act. 2019; 27:565–93.

19. Peel NM, McClure RJ, Bartlett HP. Behavioral determinants of healthy aging. Am J Prev Med. 2005; 28:298–304.
20. Teichtahl AJ, Wang Y, Wluka AE, Szramka M, English DR, Giles GG, et al. The longitudinal relationship between body composition and patella cartilage in healthy adults. Obesity (Silver Spring). 2008; 16:421–7.

21. Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015; 175:959–67.
22. Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 2012; 9:e1001335.

23. Drewnowski A, Evans WJ. Nutrition, physical activity, and quality of life in older adults: summary. J Gerontol A Biol Sci Med Sci. 2001; 56 Spec No 2:89–94.

24. Ceravolo MG, de Sire A, Andrenelli E, Negrini F, Negrini S. Systematic rapid "living" review on rehabilitation needs due to COVID-19: update to March 31st, 2020. Eur J Phys Rehabil Med. 2020; 56:347–53.

25. Carpallo-Porcar B, Romo-Calvo L, Pérez-Palomares S, Jiménez-Sánchez C, Herrero P, Brandín-de la Cruz N, et al. Efficacy of an asynchronous telerehabilitation program in post-COVID-19 patients: a protocol for a pilot randomized controlled trial. PLoS One. 2022; 17:e0270766.

26. Izquierdo M. [Multicomponent physical exercise program: Vivifrail]. Nutr Hosp. 2019; 36:50–6. Spanish.
27. Pérez-Zepeda MU, Martínez-Velilla N, Kehler DS, Izquierdo M, Rockwood K, Theou O. The impact of an exercise intervention on frailty levels in hospitalised older adults: secondary analysis of a randomised controlled trial. Age Ageing. 2022; 51:afac028.

28. Centers for Disease Control and Prevention (CDC). Telehealth interventions to improve chronic disease [Internet]. CDC; 2021 [cited 2023 May 24]. Available from: https://www.cdc.gov/dhdsp/pubs/telehealth.htm.
29. Cranen K, Groothuis-Oudshoorn CG, Vollenbroek-Hutten MM, IJzerman MJ. Toward patient-centered telerehabilitation design: understanding chronic pain patients’ preferences for web-based exercise telerehabilitation using a discrete choice experiment. J Med Internet Res. 2017; 19:e26.
30. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013; 381:752–62. Erratum in: Lancet 2013;382:1328.

31. Sun XH, Ma T, Yao S, Chen ZK, Xu WD, Jiang XY, et al. Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population Rugao longevity and ageing study. BMC Geriatr. 2020; 20:9.

32. Kańtoch E, Kańtoch A. Cardiovascular and pre-frailty risk assessment during shelter-in-place measures based on multimodal biomarkers collected from smart telemedical wearables. J Clin Med. 2021; 10:1997.

33. Heydarian H, Adam M, Burrows T, Collins C, Rollo ME. Assessing eating behaviour using upper limb mounted motion sensors: a systematic review. Nutrients. 2019; 11:1168.

34. Mirtchouk M, Merck C, Kleinberg S. Automated estimation of food type and amount consumed from body-worn audio and motion sensors. In : . Paper presented at: 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing; 2016 Sep 12-16; Heidelberg, Germany. New York: Association for Computing Machinery;2016. p. 451–62.
35. Rollo ME, Ash S, Lyons-Wall P, Russell AW. Evaluation of a mobile phone image-based dietary assessment method in adults with type 2 diabetes. Nutrients. 2015; 7:4897–910.

36. Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993; 342:1032–6.

37. Kumar P, Lee HJ. Security issues in healthcare applications using wireless medical sensor networks: a survey. Sensors (Basel). 2012; 12:55–91.

38. Hussein NH, Khalid A. A survey of cloud computing security challenges and solutions. Int J Comput Sci Inf Secur. 2016; 14:52–6.
Fig. 1.
PRISMA flow diagram. PEDro, Physiotherapy Evidence Database; RCT, randomized controlled trial.
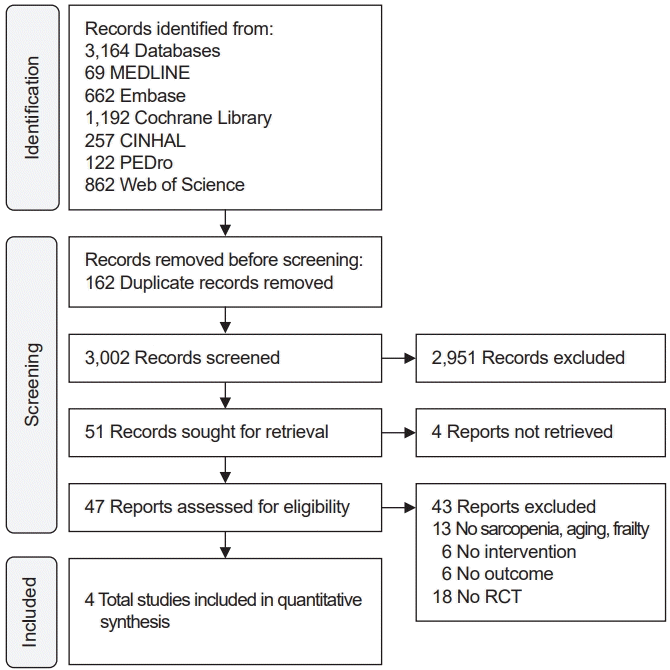
Fig. 3.
Forest plot for upper extremity strength. SD, standard deviation; SMD, standardized mean difference; 95% CI, 95% confidence interval.
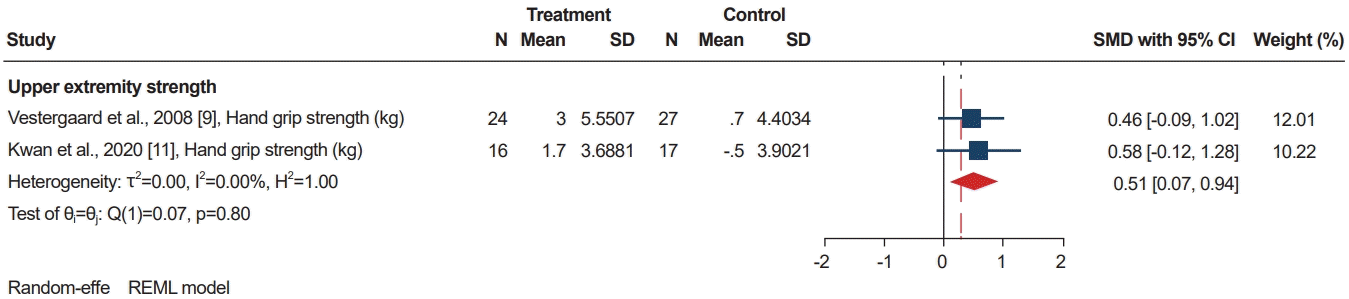
Table 1.
Characteristics and main results of the studies
| Study | Participants (IG/CG) sample | Interventions | Outcome measures | Results |
|---|---|---|---|---|
| Peterson et al., 2007 [8] | N=81 (39/42) | Telephone exercise counseling | Physical activity | In the IG, the proportion of frail was reduced by 18% over 6 months |
| Male veterans, ages 70 and over | : follow-up telephone calls biweekly for 3 months and once a month for the remaining 3 months | Physical performance | ||
| Mean age: 78.4±4.9 yr | Frailty status | |||
| Vestergaard et al., 2008 [9] | N=61 (30/31) | Home-based video exercise intervention | Hand-grip strength (kg) | Significant improvements in handgrip strength by 17.1% in the IG |
| Frail female≥75 yr | : three times a week, for five months, 60 exercise sessions | Functional ability measurements | Significant difference in the changes observed in the IG and CG in EQ-5D | |
| IG mean age: 81.0±3.3 yr | Physical performance test | |||
| CG mean age: 82.7±3.8 yr | EQ-5D (score) | |||
| Upatising et al., 2013 [10] | N=205 (102/103) | Telemonitoring case management | Fried frailty criteria | No significant increase in functional decline during the first six months (OR, 1.41; 95% CI, 0.65–3.06; p=0.38) and the latter six months (OR, 5.94; 95% CI, 0.52–68.48; p=0.15) |
| Aged 60 years or older with an ERA score of 16 or higher | : for 12 months follow-up | |||
| Mean age: 80.4±8.3 yr | ||||
| Kwan et al., 2020 [11] | N=33 (16/17) | mHealth Intervention | Hand-grip strength (kg) | Handgrip strength: improvement was significant in the IGs (p=0.009). |
| Age ≥60 years, having cognitive frailty, and having physical inactivity | : The total intervention period lasted for 12 weeks | MVPA time (min/wk) | MVPA time (median difference 86 min/wk, p=0.04; median difference 18.5 min/valid day, p=0.02) increased significantly after the intervention in the IG only | |
| Mean age: 71.0±9 yr |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download